Veeva Safety
Global Adverse Event Management and Oversight
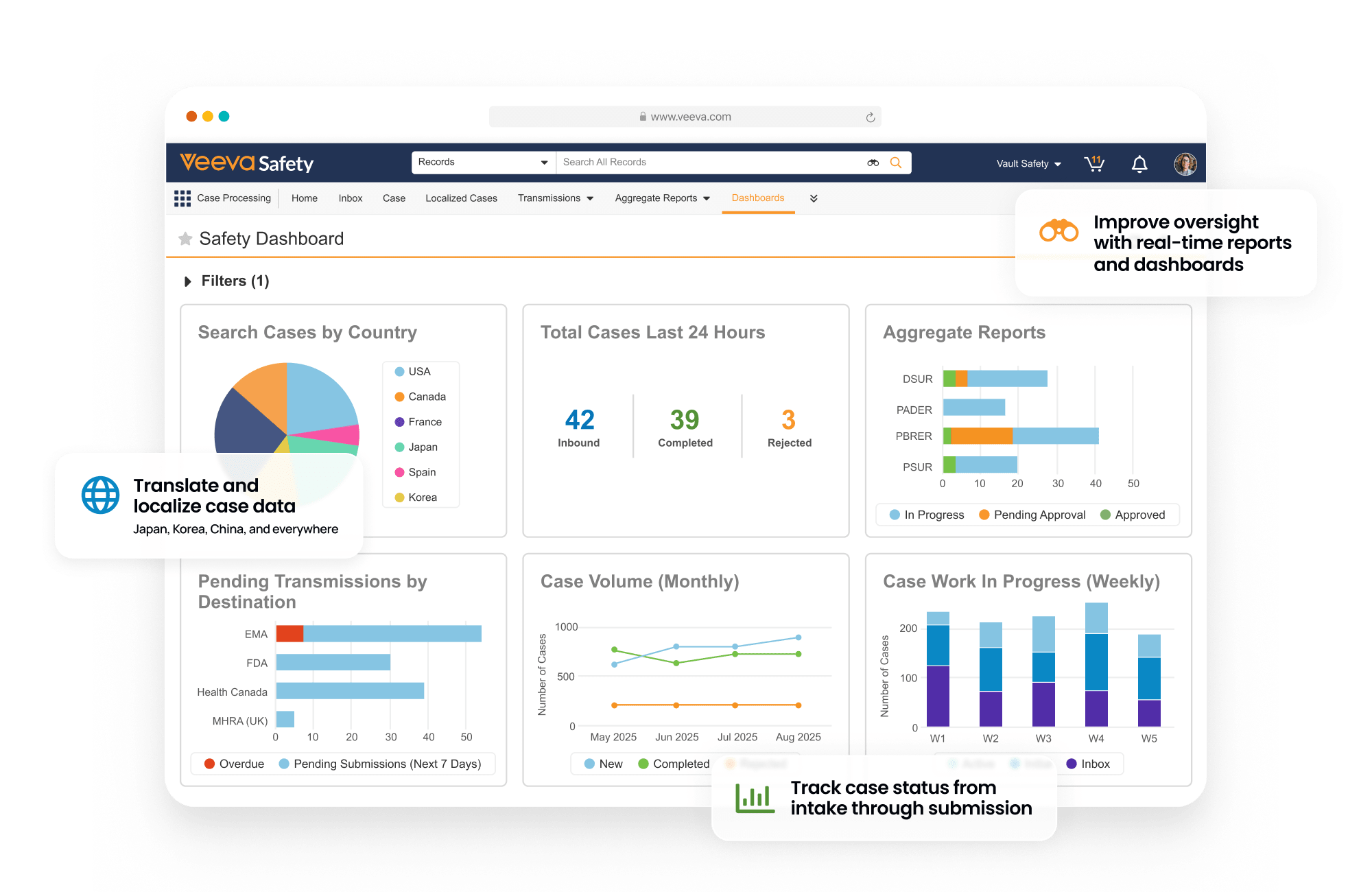
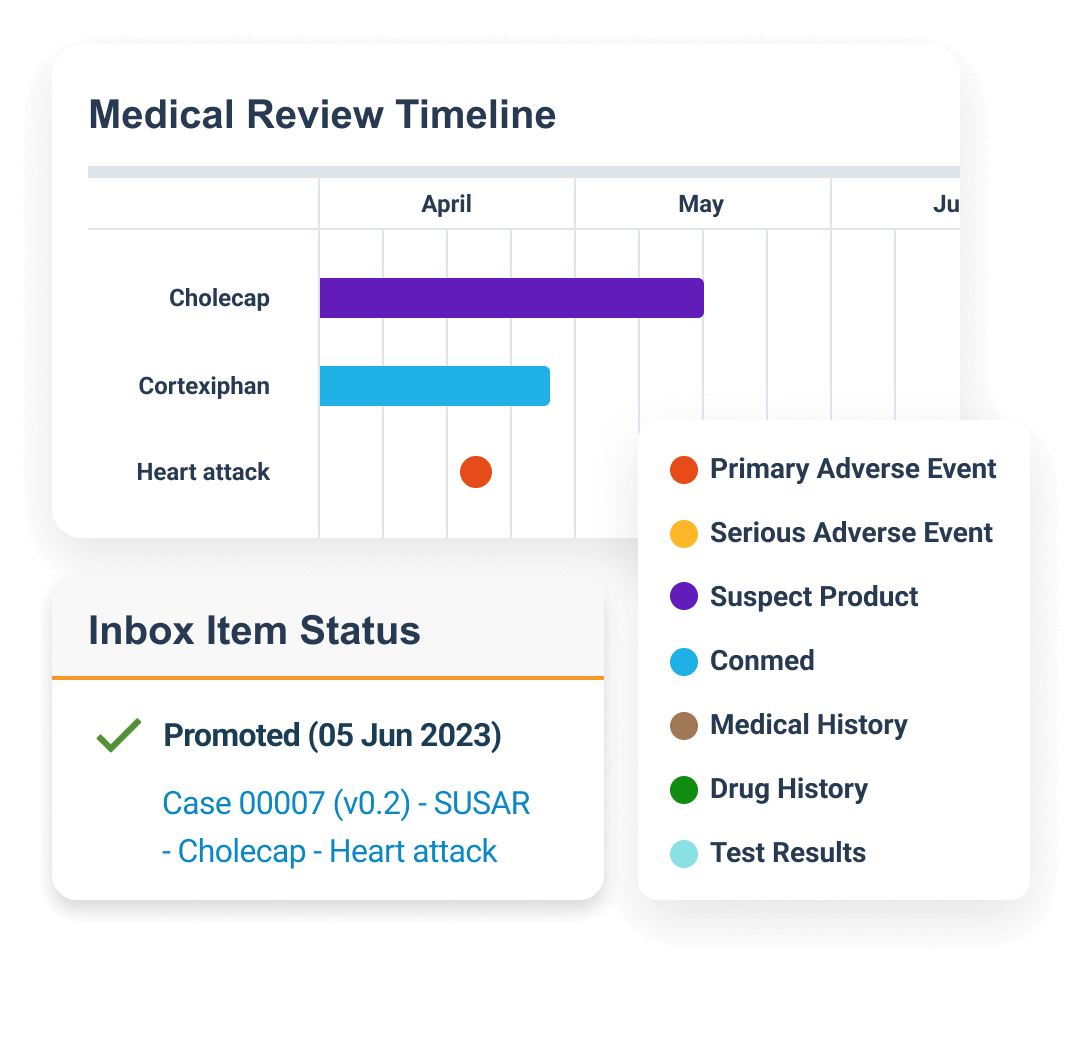
Safety is a modern individual case safety report (ICSR) management system that manages the intake, processing, and submission of adverse events for clinical and post-marketed products.
Within one system, sponsors and CROs process and manage global and domestic adverse events for drug, biologic, vaccine, device, and combination products. Built-in gateway connections and reporting rules streamline submissions to health authorities and distributions to partners.
Central coding dictionary management automates semi-annual MedDRA, WHODrug, and EDQM updates.
Hear from biopharmas transforming pharmacovigilance with Veeva Safety.
Announced 2019 Status Mature Customers 51-100
Hear how Merck streamlines processes with unified safety
Overview
Global Adverse Event Management and Oversight
Safety is a modern individual case safety report (ICSR) management system that manages the intake, processing, and submission of adverse events for clinical and post-marketed products.
Within one system, sponsors and CROs process and manage global and domestic adverse events for drug, biologic, vaccine, device, and combination products. Built-in gateway connections and reporting rules streamline submissions to health authorities and distributions to partners.
Central coding dictionary management automates semi-annual MedDRA, WHODrug, and EDQM updates.
Hear from biopharmas transforming pharmacovigilance with Veeva Safety.
Veeva AI for Safety
See in Action
Case Intake Agent
Automates case intake for faster, more accurate data extraction and identifies potential data anomalies.
Case Narrative Agent
Enhance case narratives by correcting grammar, consolidating information, and improving readability.
Why Veeva Safety
Streamline adverse event management
Customer Success
Improve safety oversight and case processing
with one global solution




Resources
Explore and Learn
Read Features Brief
Veeva Safety Features Brief

Watch Demo
Submitting ICSRs Using FDA E2B(R3) Standard
Learn More
Safety for Emerging to Growing Biotech and Biopharma

Watch Video
Streamline Package Insert Management: Veeva Safety to Veeva RIM
Read Blog Post
Improve Oversight and Data Control When Outsourcing

Read Blog Post
Building a Safety Intelligence Unit from the Ground Up

Hear How
Transform Pharmacovigilance with Veeva Safety

Watch Demo Series
See the Latest Capabilities and Best Practices in Veeva Safety

Read Case Study
Better Control of and Access to the Safety Data

