Two-Thirds of Data Managers and CRAs Warn of Data Quality Risks if Trial Inefficiencies Persist
1. Executive Summary
Pharma and biotech teams face pressure to deliver faster trial timelines under increasingly
complex protocols and constrained budgets. Meeting these demands requires not just innovation,
but a willingness to depart from ‘good enough’ processes and technology for those carrying out
clinical data tasks.
Inefficiency is the norm for many clinical professionals. Data managers and clinical research
associates (CRAs) – the people responsible for collecting, monitoring, and ensuring the data is fit for
purpose – are bogged down by manual processes and fragmented systems. Too much time is lost
to repetitive steps, re-entry of information, and workarounds outside core clinical platforms.
This research identifies the scale and root causes of that lost productivity in typical Phase III trials,
and offers a path forward. We surveyed 88 data managers and CRAs and conducted in-depth interviews
with select participants to gather first-hand accounts of the impact
of workflow inefficiencies.
Main takeaways
- Inefficiencies are embedded in workflows
Most data manager tasks take more time than they should, especially manual data reconciliation
and data review & cleaning. CRAs say updating multiple systems, writing reports, and following up
with sites all take longer than they should. - Manual work and disconnected systems are the root cause of inefficiencies
CRAs are impacted the most by system fragmentation, with data managers citing too many manual
steps and a lack of modern tool usage as the main reason for inefficiency. Most manual data
reconciliation work is happening outside of clinical systems, or using multiple different systems. - The cost of inaction is burnout and data quality
Burnout or staff turnover and data quality issues were some of the greatest consequences for
CRAs and data managers if inefficiencies are left unaddressed. CRA burnout and turnover is a widely
reported issue, but could be an increasing trend for data managers. - Organizational resistance to change hinders progress
Despite the challenges caused by inefficient processes and systems, almost half of data managers
and CRAs say that resistance to change is a key barrier to a more productive future state. A sense
of current workarounds being ‘good enough’ hides the damage caused by inefficiency in clinical trials.
Nearly half of respondents think that SOPs fail to reflect real-world workflows or underutilize available
tools, demonstrating the organizational barriers to greater efficiency. - Future roles focus on pragmatic change
AI support and risk-based approaches are anticipated to evolve data managers’ work over the next two
years, but data managers cited automation as #1 for their role evolution. The vast majority of CRAs
expect risk-based monitoring to become the norm in their roles, but feel the stretch from greater study
complexity and budget/resource constraints. - System modernization is a clear opportunity
Three quarters of data managers and over half of CRAs say their teams are actively looking for ways to
modernize trial execution. Nearly all respondents feel that better integration between clinical data and
operations systems would significantly improve execution.
“Identifying inconsistencies between two systems for the same kind of data should be easy in the
advanced tech world we live in.”Data manager’s response to the question “Why is [manual data reconciliation] so inefficient?”
2. Key Findings
2.1
Inefficiencies faced by data managers
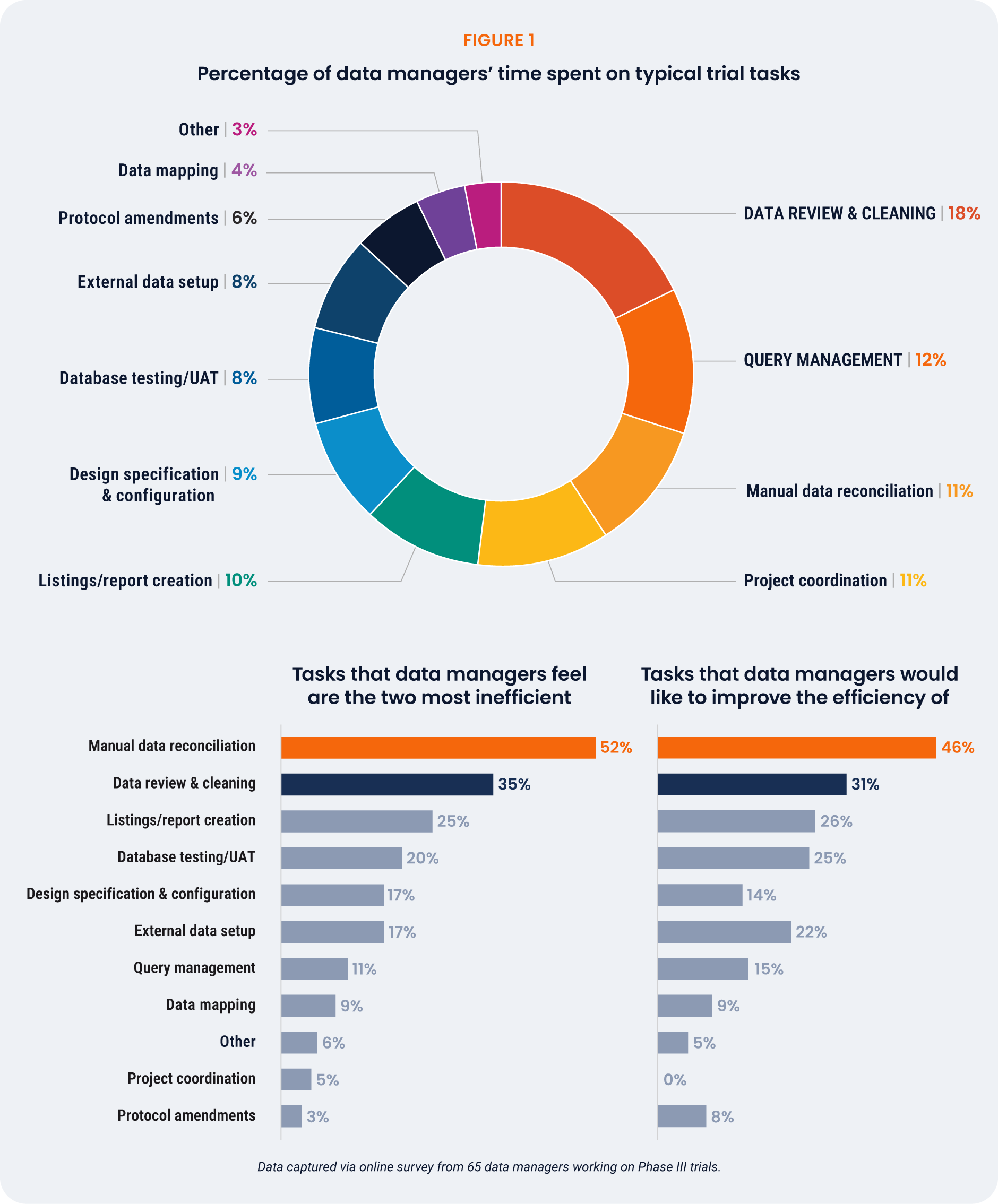
Data managers spend the greatest proportion of their
time on data review and cleaning (18%) [Figure 1].
This task is also a top inefficiency, cited as the second
most inefficient task (35%) after manual data reconciliation
(52%). Manual reconciliation occupies about 11% of data
manager’s time yet is their highest priority to improve,
indicating that inefficiency is a bigger driver of frustration
than time spent on a task.
“We have home-grown tools for
data cleaning but I don’t trust
them because they cannot
handle the variability in data
structure between studies.
Data sources have differences
in date formatting, extra spaces
in an output, column naming…
They can differ by source and by
study. There is too much variation
so we stick with Excel.”Senior Clinical Data Specialist,
Global CRO
Tasks flagged as the most inefficient (manual data reconciliation
and data review and cleaning) are also among the tasks more
often performed outside clinical systems. For those citing data
reconciliation as a top inefficiency, 97% perform reconciliations
either outside of clinical systems (50%) or with a mix of systems
(47%). For data review and cleaning, 39% of respondents perform
the task outside clinical systems, 52% with a mix of systems,
and only 9% within a single clinical system.
“It feels like for every study, we’re
reinventing what data review is.
There’s a lack of good, reusable
data cleaning programming,
which would help with both
[data cleaning and review and
manual data reconciliation].”Data Analyst, Small Biopharma
One senior data manager from a global CRO estimates that of the three hours each data manager
spends on reviewing data, one hour is wasted reviewing unchanged data, and another hour is spent
applying Excel formulas. According to this account, data managers are only spending one third of
their data review time reviewing new data.
The senior data manager explains the challenges around a lack of insight into data changes during data
review: “I complete a round of reconciliation and the next month when I download the data there is no
way to tell what data has changed. We apply a lot of Excel formulas and effort to figure out what’s been
updated and what queries got opened. Every month I pull the same reports and apply the same effort,
even though 70% of the data is the same.”
2.2
Inefficiencies faced by CRAs
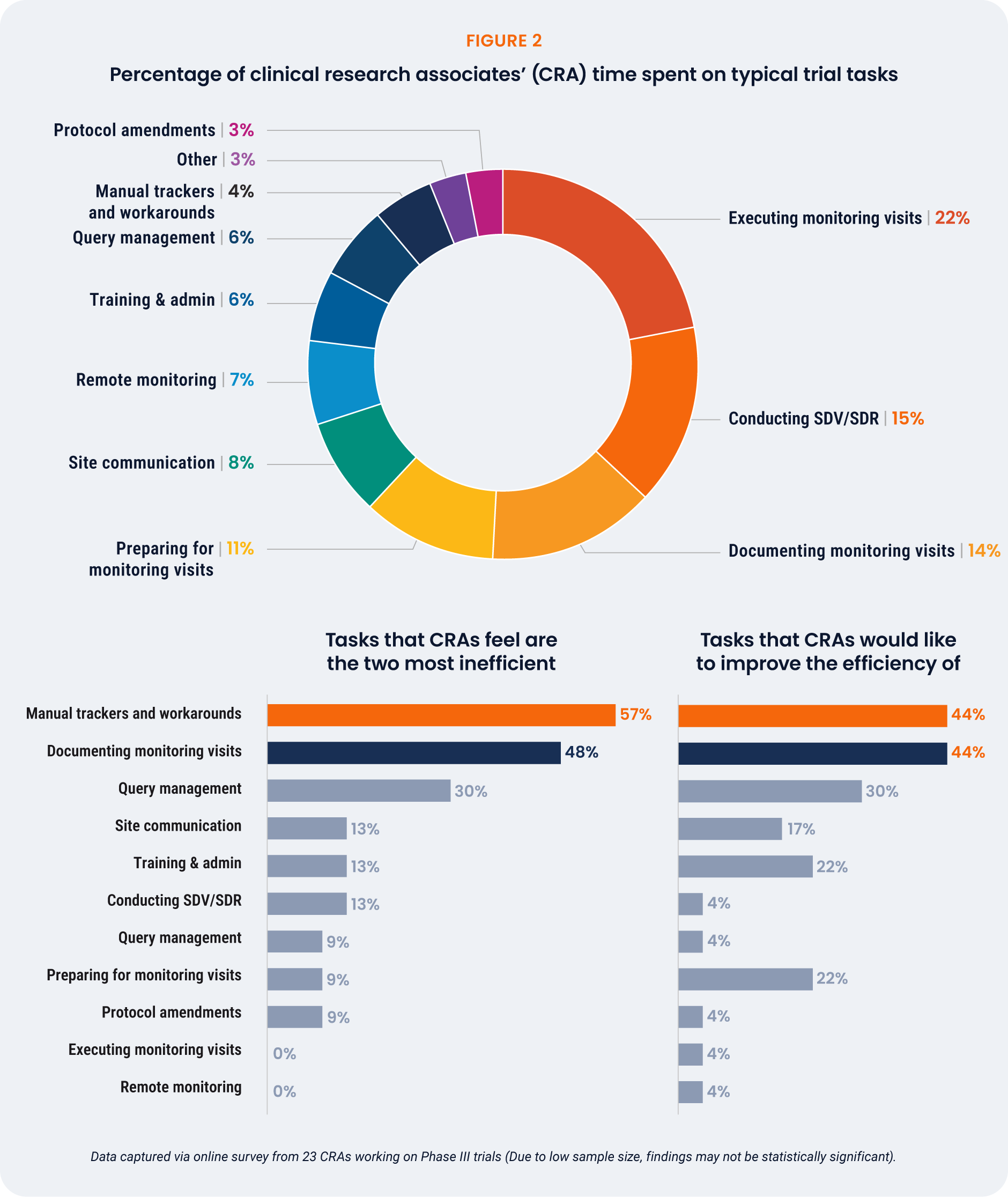
While CRAs spend most of their time on monitoring visits (22%), perceived inefficiencies center on
documentation and manual tracking – tasks that collectively consume around 18% of CRAs’ time yet
generate the highest frustration [Figure 2]. Documentation and manual tracking are identified as top
priorities for improvement by 44% of respondents.
Managing documents in multiple systems and carrying out
repetitive steps are major sources of inefficiency. One CRA from
a top 20 biopharma expresses surprise that there aren’t more
automated solutions available: “We still have to do so much manual
tracking, there’s no connection between the systems.”
Veeva benchmark analysis from over 30 biopharma companies
spanning 10 years shows that a total of 1,286 days per 1,000
monitoring visit reports (MVPs) was wasted. This equates to
$1,028,888, based on an 8 hour work day at a $100/hour rate.
Working in trackers such as Excel, OneNote, and Word accounted
for the most time wasted, costing $326,112 per 1,000 MVPs. If your
CRAs complete 20,000 MVPs, for example, the cost would reach
$6,522,240.
“I use OneNote for documenting
monitoring visits before
transcribing into the CTMS.
It’s not that I like it — I don’t have
any other tool to keep everything
in one place. I have to capture
[data] in a non-validated system.”CRA Subject Matter Expert,
Top 20 Biopharma Company
2.3
Cost of queries
Both data managers and CRAs reported that non-EDC queries take
far longer (71%) than EDC queries to resolve. Non-EDC queries
are those generated from third-party data, such as imaging, eCOA,
and central labs. On average, an EDC query takes 9.6 days to resolve,
from when it’s created to closure, whereas non-EDC queries take
16.4 days. In terms of personal effort on query management,
respondents use 34% more active time to resolve a query on thirdparty
data than a query on EDC data. This difference in resolution
time is due in part to the delay in non-EDC data reaching the data
manager or CRA, and the additional effort required to investigate
with the vendor.
It is estimated that a query costs between $28-$225 to resolve
and that, on average, a Phase III study generates 96,980 queries.
Non-EDC queries require 34% more active time, suggesting that their
cost ranges from $32.50-$300 per query. This suggests that the
cost per query is growing to $32.50-$300, and that inefficiencies in
query management – particularly non-EDC queries – reach millions
of dollars in Phase III trials.
“Non-EDC queries take longer
to resolve in part because data
managers aren’t receiving the
data frequently. When you are
querying the data it is months old.
The site has to check different
systems to find the actual reason
or value. We may also keep the
query open until the next data
transfer, to confirm whether the
query was answered correctly
or if there is a change on the
vendor side.”Senior Clinical Data Manager,
Global CRO
3. Root Causes of Unproductive Effort
3.1
Top drivers of inefficiency for data managers and CRAs
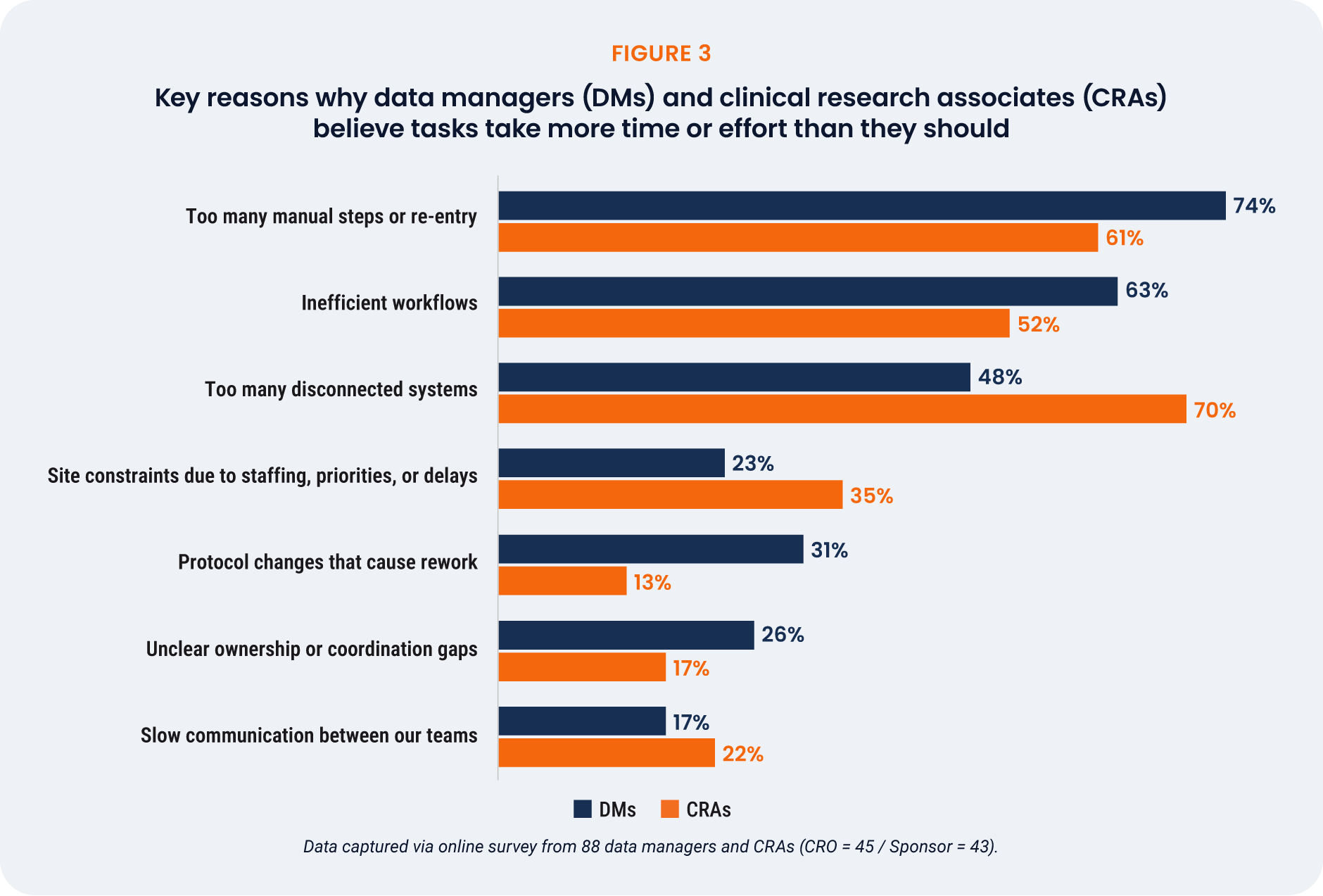
Dealing with disconnected systems is the primary cause of inefficiency for CRAs (71%). Too many
manual steps or re-entry (74%) is the main reason why tasks require extra effort and time in data
managers’ daily work [Figure 3].
One data manager’s description of why manual reconciliations are so inefficient highlights the impact
of too many manual steps on workflows: “The worst part of data reconciliation is tracking issues from
the prior reconciliation to the new data load. Most of us use Excel formulas and manually copy/paste,
use Vlookup, carry forward the comment, and then add the latest status. It is painful.”
3.2
Tools and processes as sources of inefficiency
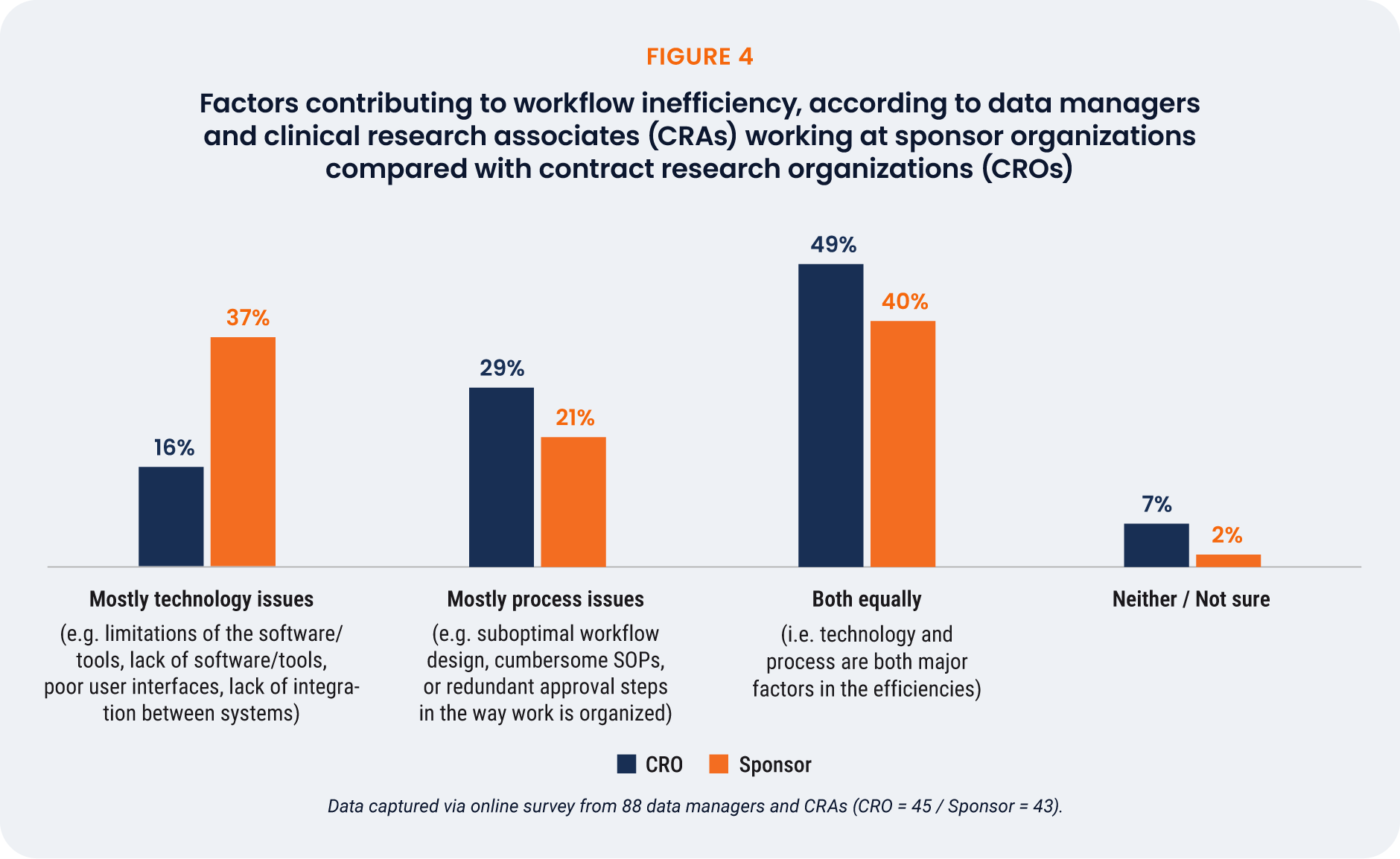
When asked whether technology issues or process issues mostly contribute to workflow inefficiencies,
or both equally, 48% of respondents said both technology and process issues equally. CRAs were slightly
more likely than data managers to point towards tech-specific challenges (30% vs. 25%), as were those
working for larger companies (>10,000 employees).
Sponsors are twice as likely to cite technology issues as the primary source of inefficiency than CROs
(37% vs. 16%), while CROs more often point to process challenges (29%) or a combination of the two
(49%) [Figure 4].
4. Implications of Lost Productivity
4.1
Risks and consequences of inaction for data managers and CRAs
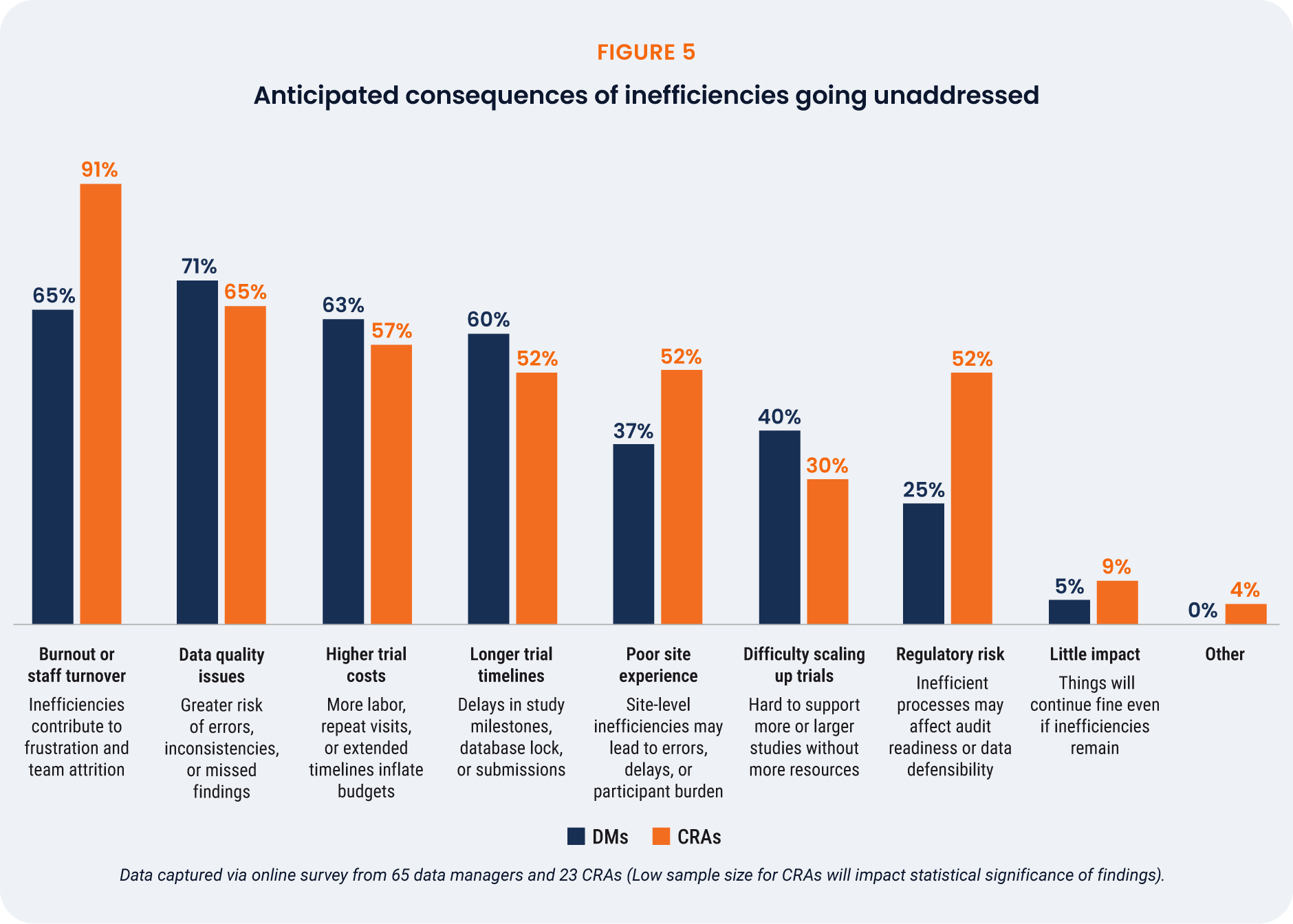
Inefficiencies in Phase III clinical trials have wide reaching impacts, on the trial itself and on those
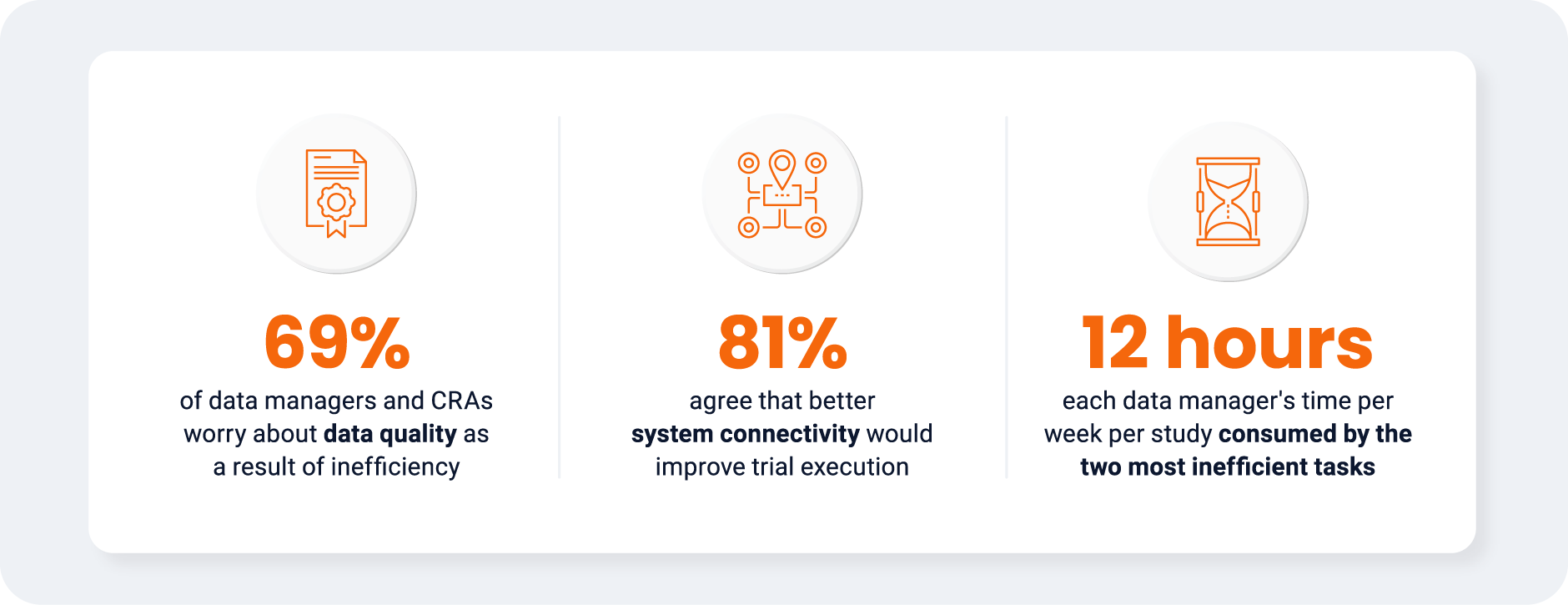
working on the studies. Only 6% of respondents believe that there will be little impact if inefficiencies
remain as they are [Figure 5]. For CRAs, the greatest concern is burnout (91%), highlighting an urgent
need to act. Clinical data managers also cite burnout as a key risk, but are most concerned with data
quality issues (71%).
As risk-based data management approaches become more mainstream, this potentially exacerbates the
threat to data quality as teams move away from reviewing 100% of the data. The significant concern over
data quality could impede any move towards risk-based reviews, as data managers may be reticent to
review anything less than 100% of the data. However, with data volumes growing and burnout risk rising,
it is untenable to continue reviewing 100% of the data. Inefficiencies must be addressed for better data
quality and data manager upskilling.
As expected, higher trial costs and longer timelines are commonly predicted consequences of inaction.
Respondents working at midsize companies are more concerned about higher trial costs (74%) than
those at larger companies (52%). These respondents are also more likely to cite regulatory risk as a key
concern (42%) compared to larger companies (26%).
4.2
Barriers to a more productive future
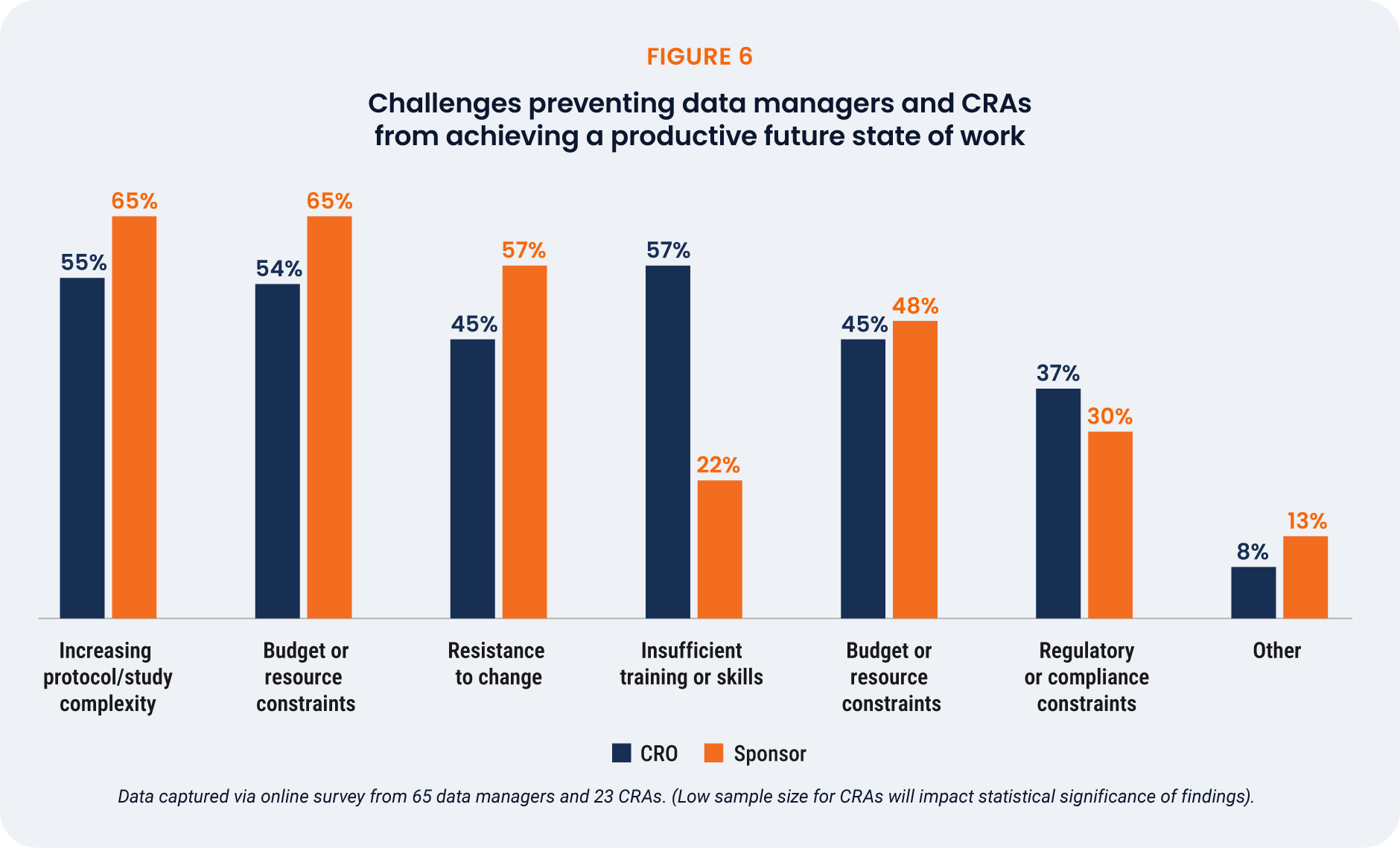
The biggest challenges to realizing a more efficient future state are
protocol complexity (58%), budget/resource constraints (57%), and
resistance to change (48%). Data managers were especially likely
to cite training gaps (57%), highlighting the need for support as
processes and technologies evolve. CRAs were concerned about
study complexity and budget/resource constraints equally (65%),
with only 22% feeling that training or skills were a barrier.
Most respondents (81%) agree that better system connectivity
would improve trial execution. Only 57% of CRAs say that their team
is actively seeking ways to modernize, compared with 75% of data
managers. However, 41% feel that SOPs fail to reflect real-world
workflows or underutilize available tools, highlighting a cultural and
procedural gap that may slow progress.
“Some trials are too complex. If I
don’t understand something as
a user acceptance tester, I have
to ask someone else which takes
more time than training would
in the long run. Lack of training
on therapeutic area databases
can also result in inadequate
testing and incomplete scenario
coverage. This can lead to issues
that require post-production
changes, potentially affecting
subject data.”Senior Clinical Data Manager, Global CRO
5. The Future Role of Clinical Data Professionals
5.1
Evolution of the clinical data manager role
Data managers have a pragmatic view of how their role will evolve over the next two years. Most expect
to use more automation for data cleaning (71%), whereas fewer think AI assistance will support them in
decision-making, completing actions, or surfacing data insights (59%). This shows that data managers
expect – and prioritize – more automation in their operations before pursuing AI assistance.
Interestingly, fewer expect their roles to shift in a more strategic direction (37%), despite the industrywide
focus on clinical data science, and only 22% think they will be involved earlier in trial design. This
suggests that data manager role evolution will be constrained if inefficiencies are not addressed first.
5.2
Evolution of the CRA role
The vast majority (83%) of CRAs expect risk-based monitoring to become the norm over the next two
years. One CRA explains their vision for a future-state:
“I would love to have a risk-based tool where the monitor can assess whether a site needs more SDV,
based on factors like number of PDs, timeliness of data activities, and number of queries. We need good
connectivity between systems, because we capture PDs in one system and EDC data in another. A good
RBQM tool needs to read across all systems to understand the state of a site.”
Compared with data managers (9%), 22% of CRAs think there will be minimal change to their role over
the next two years. AI and automation are both seen as likely to impact future roles, as is a greater use of
remote monitoring and centralized review (57% each). These shifts point to a future CRA role that’s more
tech-enabled and focused on critical risk signals, not just site visits.
6. Conclusion and Recommendations
This research highlights a clear message from clinical data managers and CRAs: an unacceptable
amount of time is spent on manual tasks and disjointed workflows. These inefficiencies are
risking data quality, burnout, and avoidable costs. It marks an executive call-to-action for change,
not complacency with past ways of working.
A notable new trend is the burnout risk among data managers – a longstanding challenge for CRAs but
a historically less reported concern for data managers. As leaders look towards increasingly innovative
data management approaches, this research reminds us that the people doing the work are not enabled
by modern technology or efficient processes. We recommend a practical approach:
- Prioritize system integration
Invest in solutions that connect clinical data and operations systems.
This will reduce manual data entry, and provide a more unified view of
trial progress. - Embrace automation
Centralize data review and reconciliation efforts in an automated tool that
directly addresses the most time-consuming and frustrating manual tasks,
within a system connected with clinical operations. See how others are optimizing these processes in this video. - Streamline workflows
Review and update SOPs to ensure they reflect real-world workflows and
fully leverage available technologies. This may involve revisiting processes
that are overly complex or rely on outdated manual steps. - Invest in ongoing training and change management
Provide adequate training and ongoing support to data managers and CRAs for
long-term adoption of new tools and processes. This will help overcome resistance
to change and ensure that professionals can effectively use new systems. - Foster a culture of efficiency
Encourage a mindset that values efficiency and continuous improvement.
This means actively seeking feedback from clinical professionals and empowering
them with the tools and processes they need to succeed.
