Simple, Standard Sponsor-Site Collaboration
Veeva Site Connect allows sponsors and research sites to collaborate on a trial by automating the flow of information to and from sites during start-up, execution, and closeout.
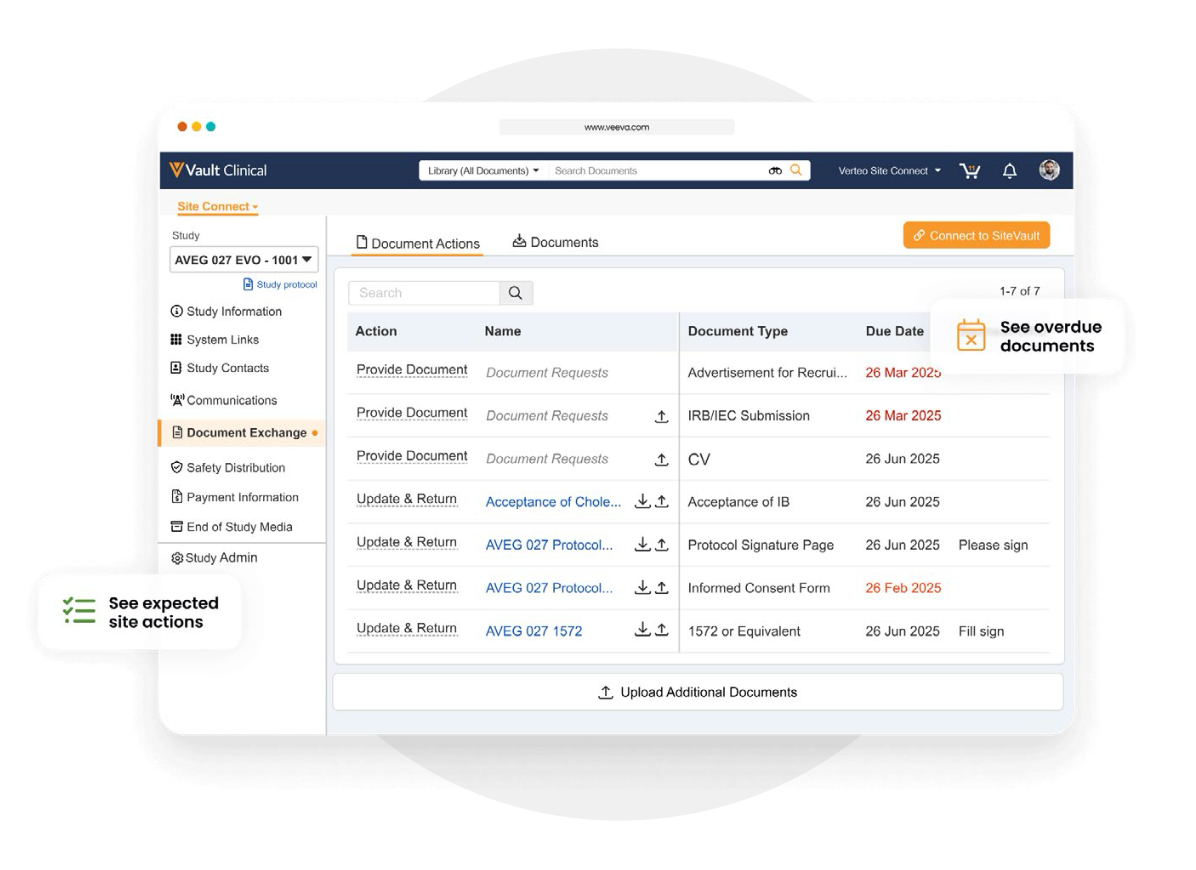
Information flow includes protocols, essential document packages, safety reports, and payment letters. Required media is sent on closeout, including completed CRFs. Information sent and received is automatically filed in eTMF.
Research sites manage tasks, documents, and data in Site Connect. Optionally, sites can connect their SiteVault for enhanced functionality.
Benefits
One system for sites and sponsors to collaborateSponsors can execute collaboration tasks across the trial lifecycle for all their sites in one application. Sites work in an optimized user interface that’s the same across sponsors, giving them a standard way to work across all trials.
Open for use by any site, anywhereSite Connect is accessible for all sites everywhere. Sites that decide to use Veeva SiteVault as their eISF get the added benefit of connecting their study for seamless bidirectional document exchange.
Faster, higher quality trialsBy simplifying and standardizing site-sponsor collaboration, sponsors and sites reduce administrative burden so they can conduct higher-quality trials at a lower cost.
Features
Document ExchangeManage and distribute site documents automatically during study initiation to speed study start-up.
System LinksStreamline site access to all study systems from a single page.
Safety Letter DistributionGuarantee delivery and tracking of important safety letter information sent to sites, and ensure principal investigators stay informed.
Study AnnouncementsShare important announcements across all sites such as deadlines, key dates, and notifications.
Payment InformationDeliver reimbursement data and payment letters to clinical research sites and receive site invoices directly within Veeva Clinical Operations.
End-of-Study MediaImport, distribute, and track final subject data — such as completed CRF output — from EDC to clinical research sites, including auto-filing in eTMF.
Study InformationAccess study information such as name, description, site number, and a link to the protocol in one convenient location.
eTMF and eISF ConnectivitySites may optionally use SiteVault so that clinical documents are automatically exchanged with Veeva Clinical Operations. This reduces manual steps associated with sponsor TMF and site ISF reconciliation activities.
Study Contacts & Site AddressesProvide sites with contact information that’s relevant for their study, such as role, name, phone, and email — and collect information from sites, including their address.
About Veeva Clinical Operations
Veeva Clinical Operations empowers clinical teams with a unified platform for efficient trial execution. Streamlining processes and improving data visibility from start-up through closeout accelerates timelines and enhances collaboration across sponsors, sites, and CROs.
