Easily meet regional regulatory requirements with a modern PSMF solution that harmonizes global content, supports local annexes, and delivers QPPV oversight.
More countries/regions are adopting EU good pharmacovigilance practices (GVP) Module II,
however, how they apply the guidance varies greatly by region. This variability makes it
increasingly difficult for sponsors to manage the pharmacovigilance system master file (PSMF).
Modern PSMF management solutions have a modular approach that reduces complexity
and overhead in maintaining multiple PSMFs while facilitating regulatory learnings between
regions. With a single global solution, real-time content visibility, and greater efficiency
in PSMF production and maintenance, safety teams can deliver more timely and higher
quality PSMFs. They can also leverage this information to improve oversight and to drive
improvements in pharmacovigilance (PV) system performance.
Harmonize management and global oversight with one modern PSMF solution
To give regulators a strong first impression of the PV system, keep your PSMF current and
available to health authorities in a timely manner. Typically, regulators will want to see the
PSMF within a week of their request.
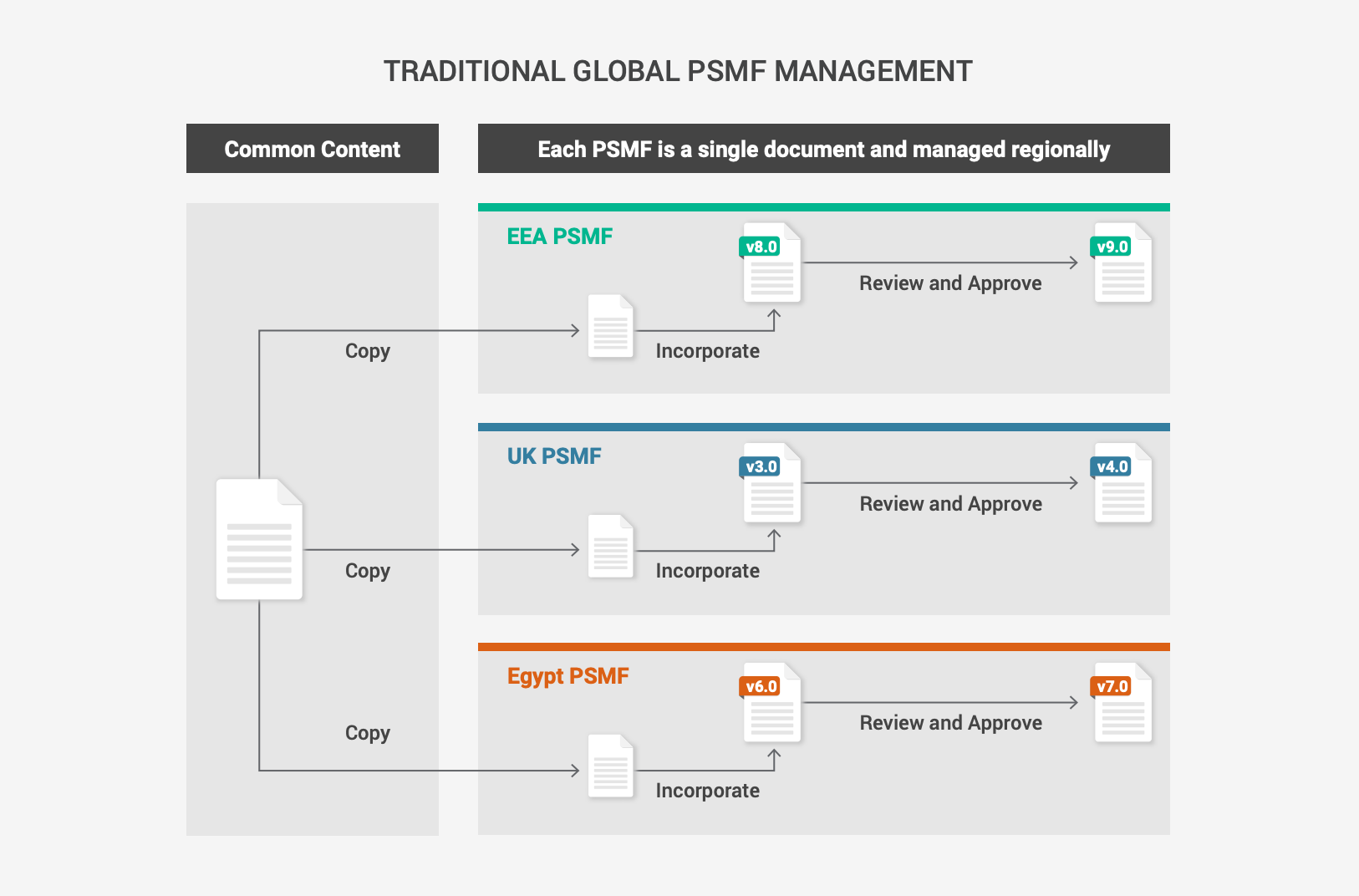
Modular PSMF to support global and regional content
Market authorization holders (MAH) often manage the PSMF at the local level, leading
to inconsistent global processes and duplicates of similar documents. Centralizing
PSMF management and taking a modular approach harmonizes content across regions.
This makes it easier to meet regional requirements while delivering global oversight. Modern
PSMF solutions provide a modifiable standard template that is organized into a global main
body and annexes, with flexibility for local regulatory and PV experts to supplement the
content with local or regional data.
Virtual PSMF binders bring together core content that is applicable to all markets with local
or regional documents and data. With one click, the safety team can generate the PSMF. Then,
they can collate the core and specific regional content to create the final document for review
and approval before submission.
Simplify the IT landscape
Managing content for all PSMFs within one validated GxP solution simplifies the IT landscape.
This:
- Eliminates the need to consolidate multiple local in-house or outsourced PSMF systems
into one global application - Reduces cost and maintenance
- Provides greater control and oversight of the content and supporting processes
Empower internal and external content owners to keep documents current
One of the biggest challenges in maintaining a PSMF is keeping content current. The PSMF
contains documents owned by different functional areas across the company and partners.
Allowing internal or external document owners and subject matter experts to collaborate,
update content, and have it dynamically reflected in the PSMF significantly reduces the burden
on the qualified person for pharmacovigilance (QPPV).
Modern PSMF solutions have flexible security models that allow companies to provide
users access to specific documents or binders and define how they can interact with them –
including editing, commenting, reviewing, or approving. Teams can set reminders to ensure
content is reviewed periodically according to standard operating procedures (SOPs), and the
QPPV team can be notified of changes to support oversight. The QPPV can also track all events
in a document’s history in detailed audit trails, including the name of the reviewer or approver
and a timestamp that can be shared with an auditor.
Automate logbook entries
Implementing a robust change control will keep your PSMF current and help you stay informed
of relevant changes and modifications recorded in the logbook. The QPPV regularly checks the
logbook for changes to maintain oversight. With a manual process, it is challenging to keep
the logbook up to date, spot which documents were changed, and identify who modified them.
Instead, look for a PSMF solution that automatically tracks changes – including the date,
nature of the change, description – and updates the digital logbook. It should also be able to
automatically notify the QPPV of PSMF content changes to help them demonstrate oversight
during an inspection and generate a more current logbook. Always having an up-to-date
logbook eliminates the manual overhead of maintaining another document and reduces
compliance risk for the QPPV team.

One-click PSMF file generation with digital QPPV approval
Traditionally, producing and maintaining a PSMF is labor-intensive and manual. QPPV teams
spend significant time obtaining and compiling documents that must be merged into a single
file for submission to a health authority.
A modular PSMF approach allows safety teams to dynamically maintain content and
automatically generate the file at any time. This includes the logbook for digital review by the
QPPV or the local person for pharmacovigilance (LPPV) before submission. Capturing the
approval of the final file with electronic signatures allows companies to demonstrate QPPV
oversight in audits or inspections. With a much faster PSMF process, QPPV teams can easily
respond to health authorities in a timely manner.
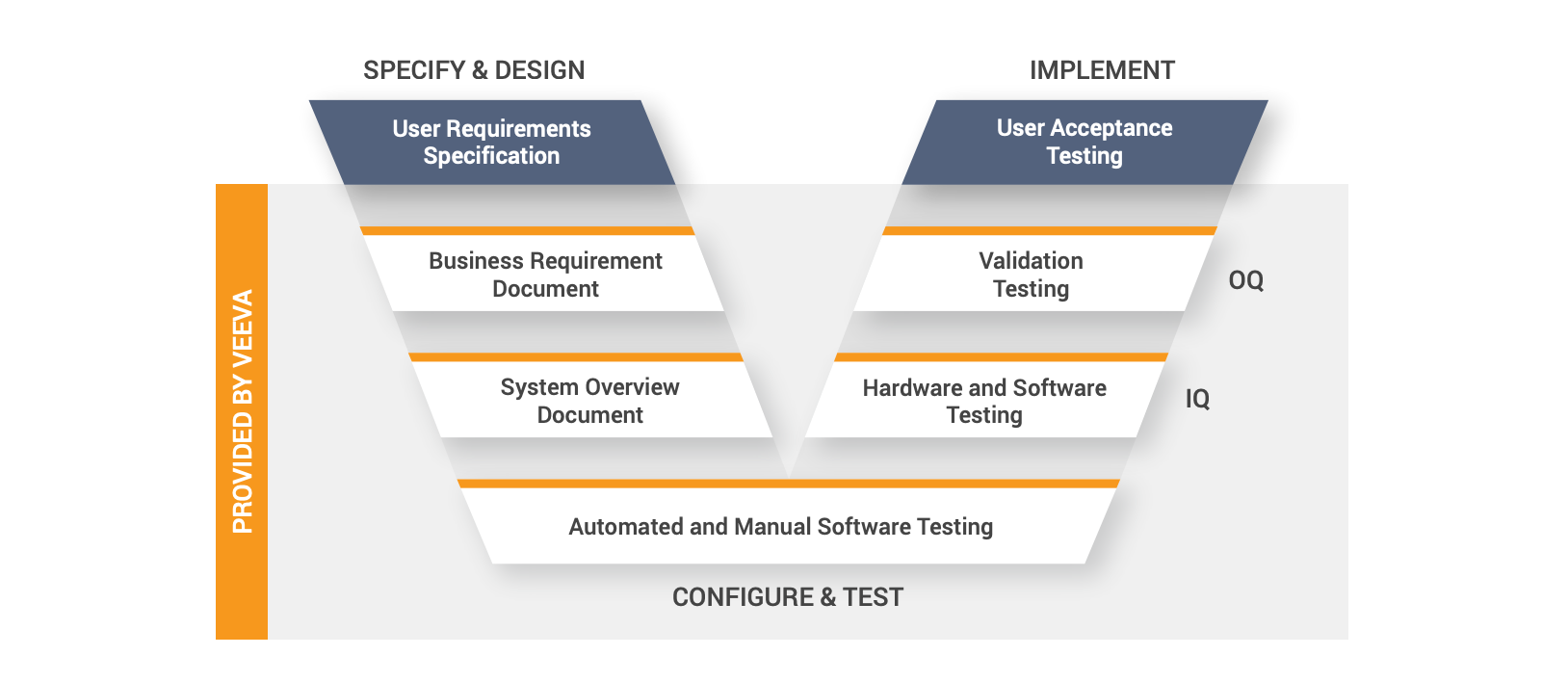
Validated PSMF management solution
Validating new software applications can be cumbersome and slow. It is even more
challenging to maintain a validated environment within a custom solution. This may cause
companies to avoid validation altogether by delaying application upgrades.
Opting for a PSMF solution designed for regulated environments will significantly reduce
overall validation time and effort. Cloud-based PSMF solution vendors perform and document
all elements of infrastructure qualification (IQ) and operational qualification (OQ) for each
major release. Sponsors can also leverage user acceptance testing (UAT) scripts and adapt
them as needed for performance qualification (PQ). Reducing the validation burden allows
even the leanest safety teams to keep up with innovation and new regulatory requirements.
Evolve PSMF into an asset for PV system performance oversight
A harmonized, collaborative approach to PSMF management will lead to:
- Greater efficiency in the production and maintenance of a PSMF
- Higher quality content
- Faster response times to regulatory authorities
- Improved process oversight
One top 10 biopharma cut their cycle time from PSMF authoring to review and approval of the
file by 30%. Standardized and modifiable templates with global and local content structures
facilitate governance and support oversight with easy navigation and efficient review. Virtual
binders enable fast generation of a specific local PSMF. “Previously, it would take me many
hours to publish a PSMF,” said a PSMF steward at a top 20 biopharma. “Now, I can produce a
merged PDF that contains all of the documents in the correct formatting in 10 minutes.”
With digital end-to-end processes, a modern PSMF solution provides greater control and
continuously collects data to enable compliance tracking. For example, automated emails notify
content owners of reviews and updates. QPPV teams can leverage reports to track whether
content tasks are completed on time. With greater transparency, “We’ll start to see much more
engagement from the PSMF contributors when it comes to completing their tasks,” said the top
20 biopharma PSMF steward. Process data can support embedded analytics for the QPPV team,
either leveraging existing reports and dashboards or modifying and creating new ones. “Reports
give great oversight of the PSMF. You can see what stage in the workflow documents are in,
or which contributors still have open tasks and reach out to them,” the PSMF steward said.
Cloud-based solutions are not only transforming PSMF management. They are also changing
how content is managed across biopharmas and their external partners. For example, sources
for PSMF documents can be owned by departments outside of safety. This causes difficulties
identifying content owners and verifying that content is current. With a single cloud platform
across functional areas, PV employees can associate documents in any department with a
PSMF – eliminating content duplication and ensuring data integrity.
As companies adopt modern PSMF solutions and technologies continue to advance, the PSMF
can evolve from a repository of content to an asset for enabling real-time oversight of PV
system performance. With greater operational visibility, the PSMF can drive proactive focus
into key areas to improve performance and reduce inspection requests and time on site.
Hear how Moderna simplified PSMF management for better compliance and efficiency.
