Over the last decade, emerging biotechs have branched out from historically
common licensing, partnership, and acquisition paths to launch products
themselves. In fact, industry data estimates that by 2026, first-time biotech
launches will outnumber those from established companies by more than
two to one.
For many emerging biotechs, preparing for launch means focusing attention
on essential foundational content areas. The quality and compliance of
the foundation you lay for your content workflows now — including medical,
legal, and regulatory (MLR) review processes — can create a competitive
edge and help your organization scale more efficiently in the future.
Compliant content management optimizes review and approval
Speed is critical to maintaining launch timelines, but moving too quickly
can lead to costly mistakes. In 2023, life sciences companies in the
U.S. paid roughly $1.8 billion to settle False Claims Act violations and
off-label promotions.
The right foundation for MLR review and approval reduces the risk of
fines while increasing speed and efficiency. Along with improved
compliance through a clear chain of custody and automated audit trails,
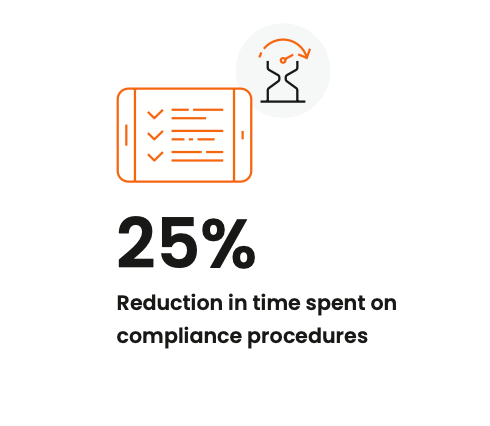
Veeva PromoMats users reported a 25% reduction in time spent on
compliance procedures.
“We’re trying to be transformative, but we have to lay the foundation first.”
Robert Yee, Senior Product Manager, Azurity Pharmaceuticals
Fast-track MLR with a dedicated system
Emerging biotechs commonly use email and spreadsheets as a starting
point to manage MLR reviews and approvals. However, these systems
are highly manual and time-consuming, so it’s easy to lose track of
where an asset stands in the review process. The result: a drain on your
organization’s limited resources and increased compliance risks.
A more sustainable option is to automate these cycles through a
dedicated MLR review system. With review and approval tracked in one
place, your stakeholders can manage approval queues and gain visibility
into content moving down the pipeline. Meanwhile, you can quickly
identify and address any bottlenecks in the process. Organizations with
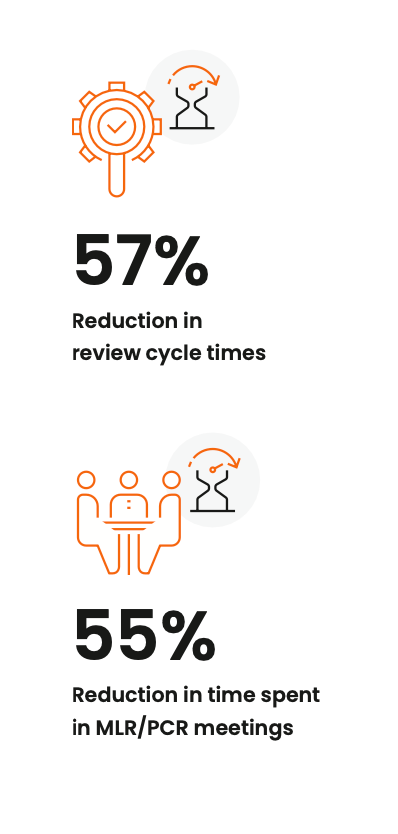
streamlined MLR processes in Veeva PromoMats report a 57% reduction
in review cycle times and a 55% reduction in review meeting duration,
advancing speed and compliance.
Modern claims management enables launch efficiency
Manual processes like tagging and anchoring claims to supporting
references are not only time-consuming but also prone to human error.
As you navigate peak content volumes and aggressive deadlines
during launch, there’s no room for delays. A modern approach to claims
management can help your organization avoid slowdowns and maintain
compliance with the automation of repetitive tasks, clear audit trails,
and traceability.
“By using a core technology solution, we have a single source of truth for approved content and the ability to flex and optimize our MLR workflows as the company evolves.”
Martin Boyle, Senior Director of Field and Marketing Operations, Regeneron
Use modern claims management to optimize your content operations
by automatically tagging and linking materials to approved claims.
And when you stand up a central claims library, your stakeholders gain
access to standardized language, improved transparency, and accurate
documentation.
“80% of our claims are now linked automatically. This automation has significantly cut down manual linking, which used to take 10 minutes per claim, and now constitutes only about 20% of the overall process.”
Senior Marketing Director, Emerging Biotech
Automate eCTD submissions and build a single source of truth
Navigating eCTD submissions can be complex, but it also offers a
chance to create significant efficiencies from the start. As you define
your organization’s process for eCTD submission, automating certain
steps will save time and resources in the long term. PromoMats users
can automatically generate submission-ready forms and bulk generate
compliance packages consistent with the latest eCTD requirements.
There’s also an opportunity to build a single source of truth for submissions.
Using multiple different solutions (or none at all) to create an eCTD
submission package, approve it, and submit it to the FDA creates confusion,
redundancies, and re-work. A unified platform like Vault streamlines the
promotional material submission process by reducing data redundancy and
compliance errors.
Organizations that have implemented modern claims management using PromoMats report:
- Faster time to approval by utilizing pre-approved claims
- Reduced time and budget spent on creating and linking/annotating references on reused content
- Improved quality of MLR submissions and increased confidence in review teams
- A foundation ready for future content expansion
“Operating as a small team with limited resources, we needed a compliant way to simplify digital content development and keep our operations agile as we grow. Automating claims management streamlines MLR reviews to speed content turnaround for approvals.”
Dana Smith, Senior Director, U.S. Patient Marketing, Calliditas Therapeutics
Anticipating and preparing for future challenges
Establishing a standardized content taxonomy from the start
Emerging biotechs that implement a standard taxonomy while building a
content foundation can avoid future disconnects with channels, intent,
and format. Veeva announced a freely open and available Commercial
Content Kernel to help software applications, data products, and people
talk to each other with greater consistency and accuracy. It provides a
standard content hierarchy, classification, and description for organizing
and delivering content.
As an emerging biotech, adopting the Commercial Content Kernel from
the start will help you increase speed, efficiency, and quality of data
management for your organization in the future. When content operations
scale, you’ll be able to locate, manage, and repurpose materials much
more easily and efficiently.
Adopting a comprehensive, connected solution
Traditional content methods — siloed systems or paper-based processes —
can delay content availability and create compliance risks. The integration
of PromoMats with other Veeva solutions, such as Veeva RIM, Veeva
Medical, and Vault CRM, creates a connected ecosystem that enhances
content management across the end-to-end lifecycle.
“With Veeva PromoMats, we can easily collaborate, share, and integrate digital content across
our Veeva ecosystem. This drives success right out the gate because we immediately gain valuable insights to inform more efficient content production and can implement changes quickly, as needed.”
Associate Director, IT Product Manager, Biotech
These connections allow for seamless data flow between different systems,
ensuring that content is compliant and of the highest quality throughout
creation, review, approval, and distribution.
Schedule a call with Lizzy Ross, Senior Manager, Commercial Content Strategy, Veeva, to discuss your content strategy, and learn why 350+ emerging and mid-size biotechs use PromoMats to build a compliant content foundation.
