Blog
Continuous Publishing Best Practices to Increase Regulatory Efficiency
Jan 13, 2025 | Rachel Kelly
Jan 13, 2025 | Rachel Kelly
Streamlining submission processes is central to keeping pace with evolving global health authority requirements and speeding products to market. Continuous publishing on a unified platform drives efficiency by consolidating data silos, enabling parallel workflows, and enhancing cross-functional collaboration. However, unifying regulatory operations and adopting continuous publishing depend on more than just a technology solution. To accelerate submission timelines, companies must take an enterprise-wide approach to harmonize functional processes around more involved publisher and authoring roles as well as become more data-oriented operationally.
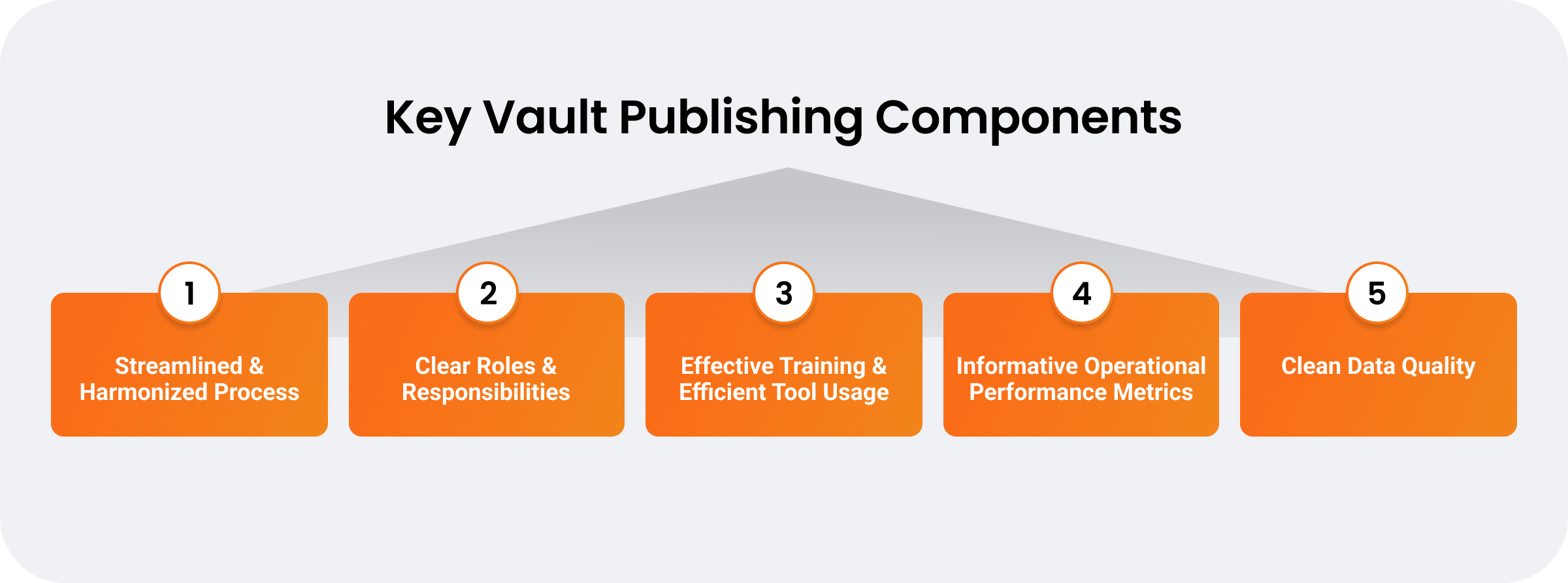
Drive integrated regulatory processes and functions
In 2022, Pharmanovia implemented Veeva Vault Submissions Publishing to accelerate submission cycle times, enhance visibility, and increase document quality.
During deployment, the team learned that transitioning to continuous publishing is not a one-time shift, but an ongoing journey. Pharmanovia developed an integrated strategy to inform plans and decisions, preparing them for unexpected challenges along the way. Early stakeholder engagement and cross-functional alignment were key to harmonizing processes, ensuring top-down transparency, quality, and collaboration.
Continuous publishing is more than automation and data centralization — for success, it requires an integrated, end-to-end approach to business processes and functions.
Adapt operating models to meet evolving roles
With continuous publishing, authors take greater accountability for document quality and leverage automation to reduce manual editorial tasks. As a result, publishers are elevated to a more strategic role, focusing on submission coordination and cross-functional alignment. Companies adopting continuous publishing are reassessing operating models to proactively address potential challenges that come with changes to the author and publisher roles.
Nordic Pharma adopted continuous publishing in 2024 after consolidating fragmented legacy systems onto a unified Vault RIM platform. The company merged submission management with business administration functions to form a dedicated RIM database management team. The goal is to establish a foundation for sustainable operational efficiency by expanding the database management team’s role in publishing. This includes involving publishers to provide technical support for validation errors, ensuring a smoother transition to continuous publishing.
Support adoption through continuous change management
For seamless adoption of new systems and processes, effective training must cover tool usage and practical applications. A continuous support plan — including leveraging resources like Veeva Vault Submissions Publishing eLearning — is critical to a successful change management strategy. Highlighting benefits for end users, alongside broader business value, fosters greater engagement and understanding of the reasons for change.
To support the transition to continuous publishing in 2024, one global biopharma established a Submissions Enablement Team as subject matter experts for evolving health authority requirements. Testing complex scenarios over six months prior to going live, the company enhanced end-user proficiency, satisfaction, and efficiency. Effective training in combination with Veeva’s resources significantly reduced downstream process issues in data entry accountability, ownership, and accuracy. To further support end users, the company deployed a generative AI chatbot to answer common “how-to” system questions.
Ongoing monitoring of validation errors, data quality, and user feedback allows the organization to identify and address emergent issues for continued support and process improvements.
Define operational performance metrics for data-driven decisions
Many biopharmas, regardless of size, struggle to define impactful operational performance metrics. However, establishing clear KPIs is key to data-driven decision-making for submission management processes and identifying underlying issues.
One global biopharma developed metrics based on its business case value drivers to address data accountability, ownership, and accuracy challenges. By focusing on strategic, operational, and data quality metrics, the company located problem areas and implemented targeted solutions.
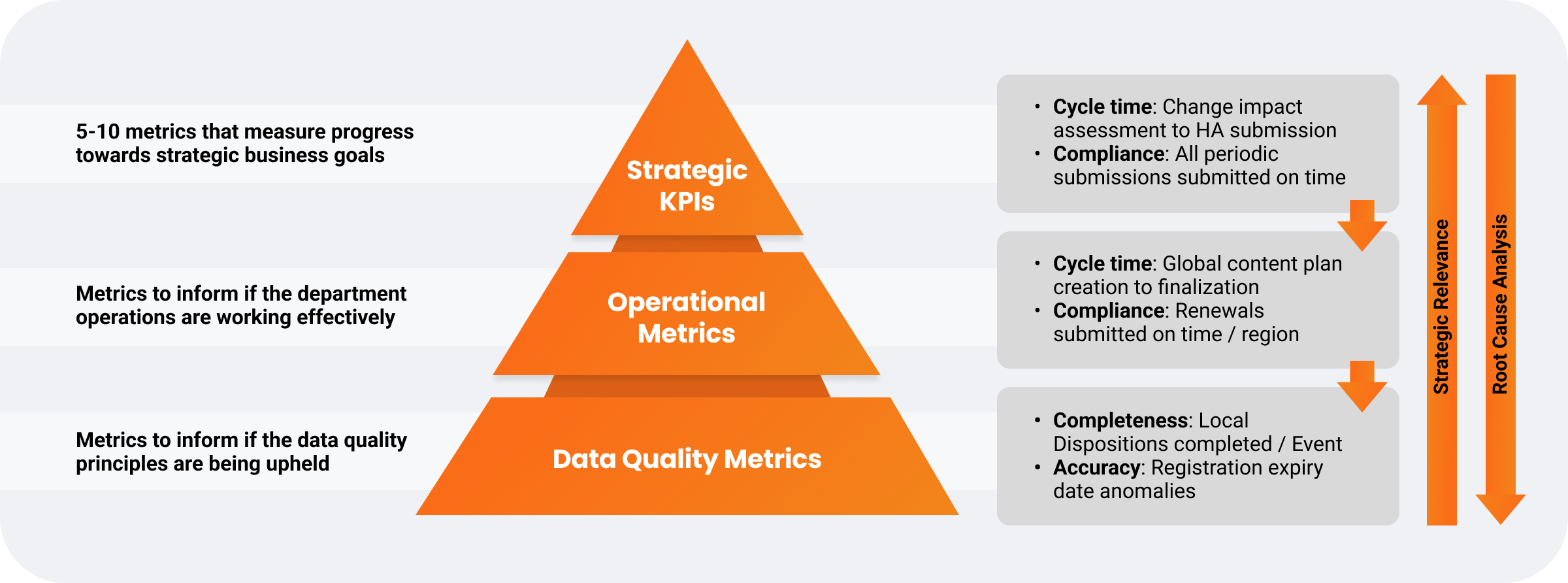
Operational performance metrics measure the value of improvement initiatives, provide visibility into roadblocks, and support workload forecasting through real-time submission tracking reports and dashboards. Aligning metrics with value drivers enhances team resource management and planning.
Build a data-centric regulatory organization
Biopharmas are adopting a data-centric organizational approach to improve data ownership, accountability, and quality. Mapping end-to-end data flows and prioritizing quality before implementation helps prevent downstream issues.
Merck KGaA introduced a pre-submissions checklist after implementing continuous publishing to enhance data entry accountability and quality for event details and joins. Taking two minutes to improve data quality at the event level can save two hundred minutes correcting issues downstream.
Building a data-first culture, where data quality is a regular part of performance reviews, reinforces enterprise-wide data accountability and ownership. Defining and reporting on data quality metrics enhances visibility, driving consistency and confidence throughout the organization.
Maximize value of continuous publishing through an integrated approach
Continuous publishing transforms regulatory operations by leveraging automation and data centralization on a unified platform to accelerate submission timelines. However, industry leaders recognize that success relies on more than technology alone.
Realizing the full business value of continuous publishing requires aligning processes and roles, and ensuring ongoing end-user support. Defining clear performance metrics strengthens data accountability, increasing data quality and consistency. With an integrated approach, organizations can drive sustained operational efficiency and maximize the value of continuous publishing.
Watch how Veeva Vault Submissions Publishing eLearning helps organizations understand and implement best practices for continuous publishing.