Veeva Study Training
Streamline and Automate Study Training
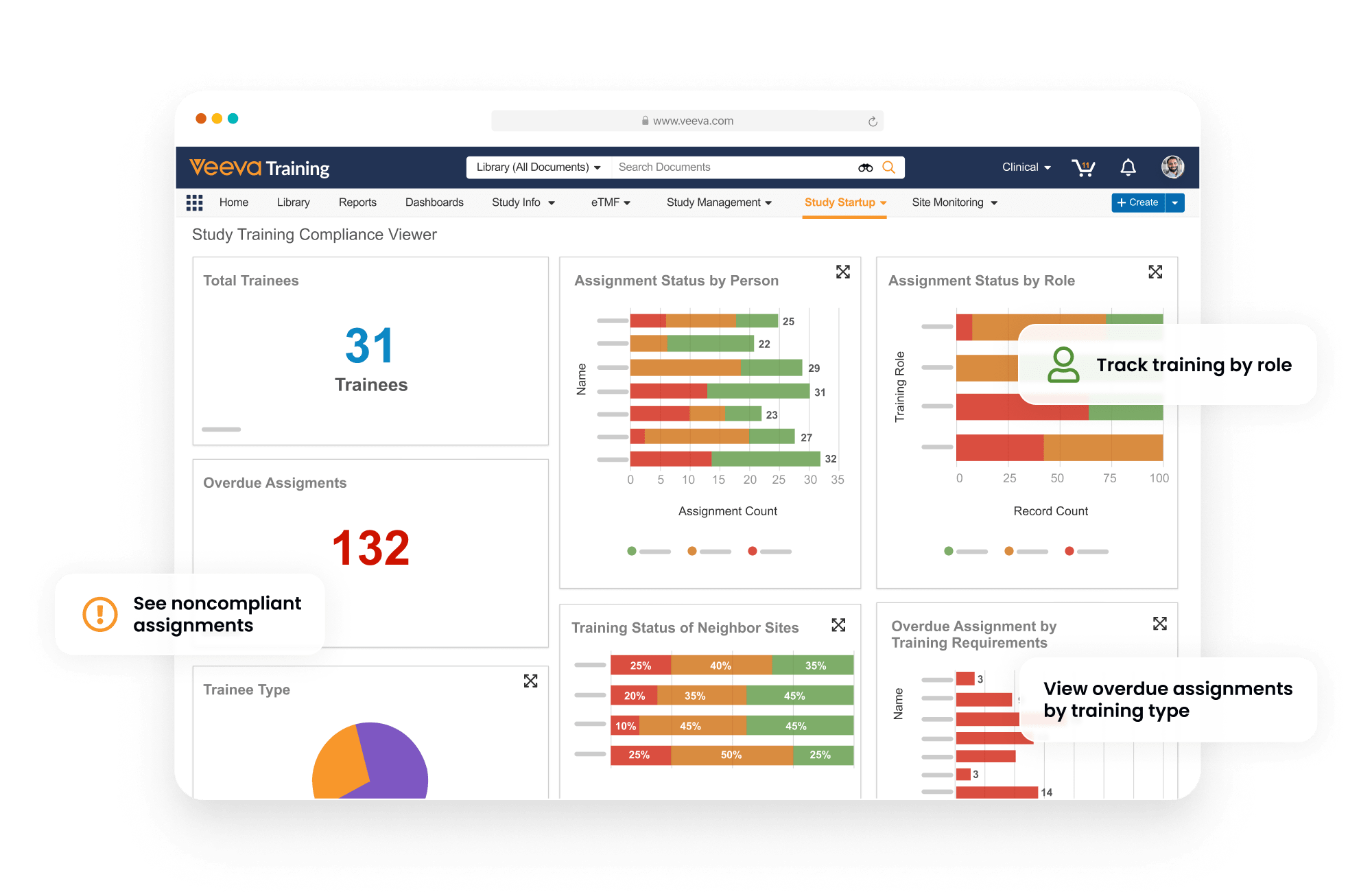
Study Training manages GCP and study-specific training for research sites, CROs, and sponsor personnel. It provides document, video, and SCORM/AICC training, in addition to quizzes and classroom capabilities based on curricula and training requirements.
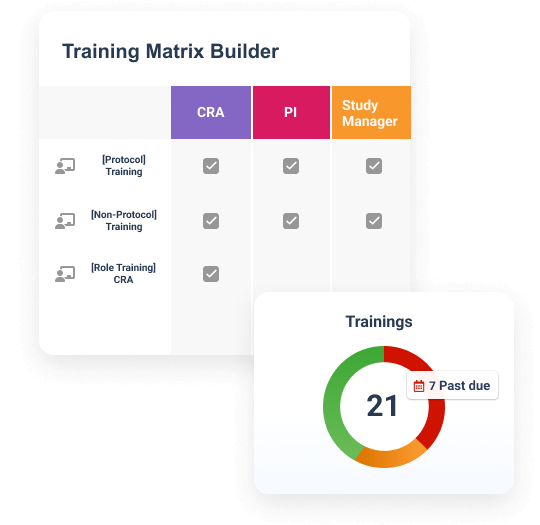
Teams can create a protocol-specific training curriculum, which automatically assigns training based on a user’s role, responsibilities, and location. Completed training is documented automatically in an inspection-ready format for study teams and CRAs to leverage.
Study Training connects to eTMF to eliminate the need to manually capture study and site information.
Announced 2022 Status Mature Customers11-50
See how Sanofi projects a 30% study training cost reduction
Overview
Streamline and Automate Study Training
Study Training manages GCP and study-specific training for research sites, CROs, and sponsor personnel. It provides document, video, and SCORM/AICC training, in addition to quizzes and classroom capabilities based on curricula and training requirements.
Teams can create a protocol-specific training curriculum, which automatically assigns training based on a user’s role, responsibilities, and location. Completed training is documented automatically in an inspection-ready format for study teams and CRAs to leverage.
Study Training connects to eTMF to eliminate the need to manually capture study and site information.
Why Veeva Study Training
Efficient and compliant training
Resources
Explore and Learn
Read Features Brief
Find Veeva Study Training Features to Streamline and Automate Training
Read White Paper
Discover a Practical Guide to Improving SIVs and Site Training

Watch Demo
See Veeva Study Training in Action
Watch Customer Video
Bayer Streamlines Study Training and Enhances Oversight

Watch Customer Video
Cerevel Drives Efficiencies and Reduces Compliance Risk

Watch Demo
Impact Assessments Show Document Modifications for Updated Training