Features Brief
RIM-PromoMats Connection Features Brief
To help Vault users comply with mandatory electronic promotional material submissions to the FDA, Veeva developed the RIM-PromoMats Connection. This enables regulatory and commercial teams to automatically share critical information within Vault.
How it Works
First, organizations need to configure the eCTD compliance package feature in Veeva PromoMats. Next, users create a preclearance compliance package or 2253 submission package (with or without linked references) using the eCTD compliance binder template. Once they move the compliance package to “ready for submission”, it triggers the RIM-PromoMats Connection to create a submission within Veeva RIM. That submission creates a content plan, adds all relevant cross-links, and matches those cross-links to the content plan. If licensed, the content plan can then be published through Veeva Submissions Publishing.
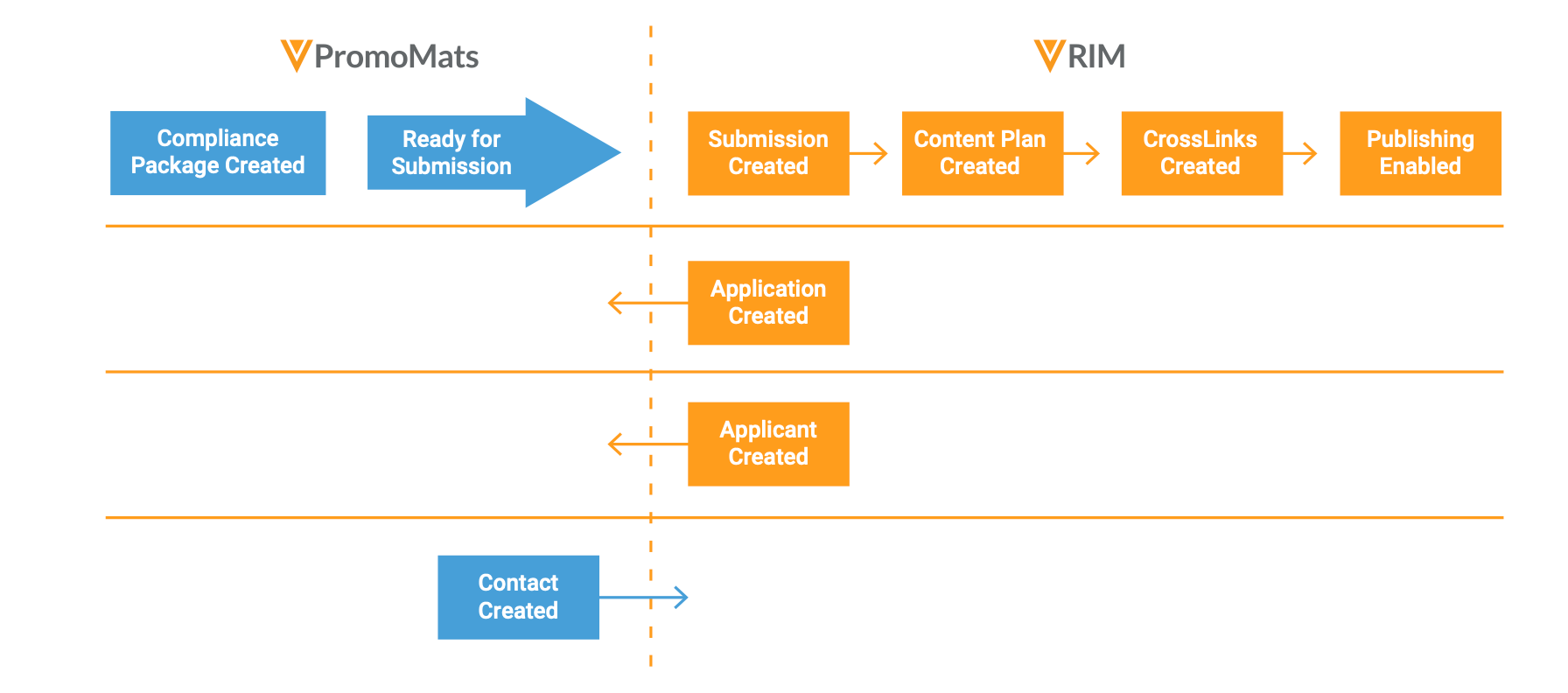
As was previously the case, Veeva RIM serves as the source of truth for the application and applicant, whereas Veeva PromoMats maintains the promotional labeling and advertising contacts. However, now users have the flexibility to create submission activities related to their promotional materials through a seamless integration.
Benefits
-
With the RIM-PromoMats Connection, regulatory and commercial teams can streamline the promotional material submission process by reducing data redundancy and compliance errors.
-
Users can automatically generate submission ready documents for materials, references, and labeling including converting Vault link annotations to relative PDF links, which saves time and manual effort.