Features Brief
Veeva Clinical Data Features Brief
Today’s clinical data journey brings new complexity and effort. Patients, sites, sponsors, and CROs are surrounded by more data, and too often, technology adds burden.
To advance better science together, we must simplify and standardize.
The Veeva Clinical Data platform simplifies data collection and processing for all users. A connected data foundation enables end-to-end processes across study teams and Veeva Development Cloud.
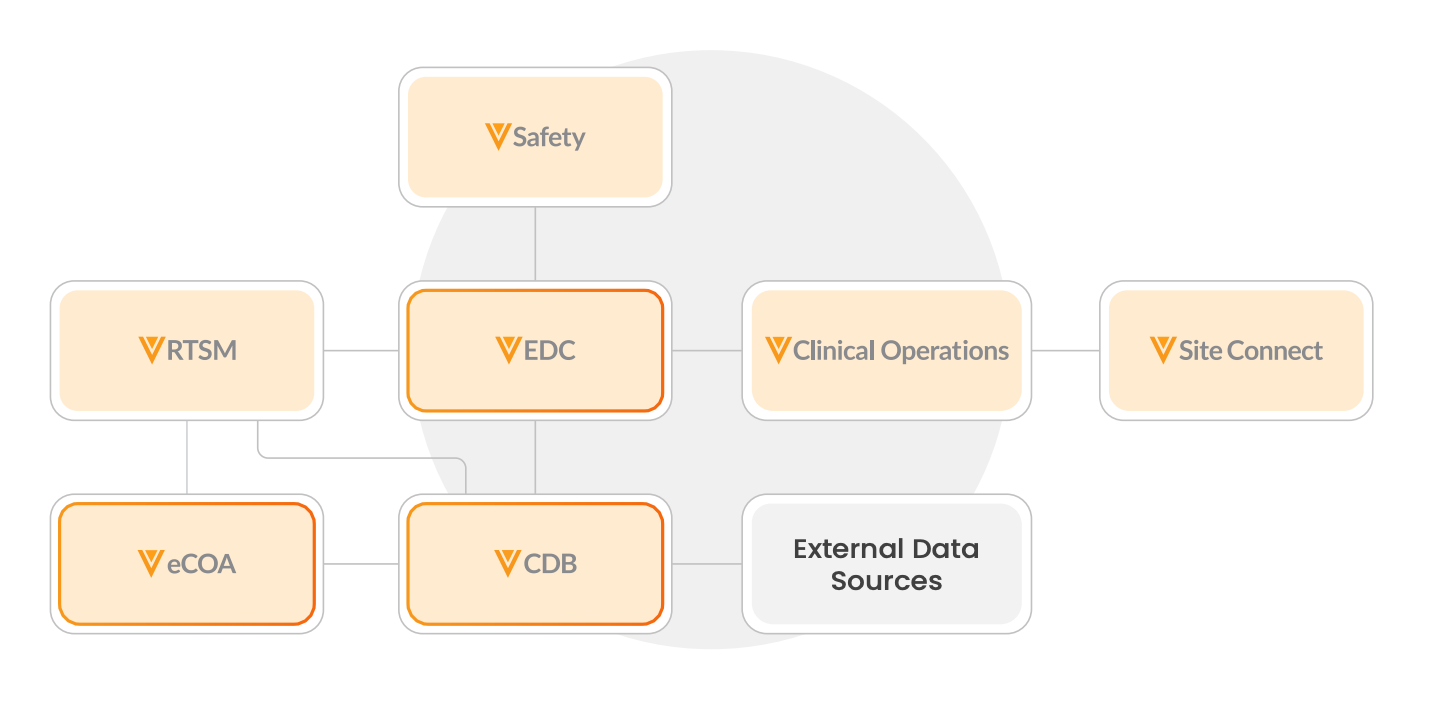
Business Benefits
-
Simplify trial participation for patients.
With Veeva eCOA, patients access trial documents and complete all surveys (and consent forms) in a single app. This app works on any device and across all studies.
-
Reduce effort for sites.
Veeva EDC helps users focus on action, with features like dynamic casebooks and pending actions. VeevaID gives clinical researchers a single sign-on experience.
-
Improve speed and efficiency for sponsors.
Veeva CDB gives data managers a single place to aggregate and clean all study data. Vault platform connections automatically transfer key data between Veeva EDC, Veeva CTMS, and Veeva Safety.
Veeva EDC
Veeva Electronic Data Capture (EDC)
Provides an end-to-end environment to collect, review, and process trial data about patients.
- Studio design environment to build patient forms (including edit checks) without the need for custom programming. The drag-and-drop interface comes with reusable templates and a standards library. Innovative features such as dynamic visits and forms and self-documenting specs allow studies to be built quickly.
- Centrally collect local labs and manage medical coding. Govern local lab units and reference ranges in a single master list.
- Real-time and risk-based UAT to eliminate delays. A system-generated Study Differences Report documents all changes.
- Apply amendments without downtime or migrations, even for sites.
- Quality controls include querying, targeted source data verification (T-SDV), and protocol deviations.
- Data lock and post-processing features to close out studies, including end-of-study media distribution and archiving.
Veeva CDB
Veeva Clinical Database (CDB)
Aggregates, cleans, and transforms clinical data from multiple sources, including third-party EDCs.
- A durable ingestion engine eliminates fragile custom integrations. Inbound vendor data is automatically checked for proper formatting and accuracy.
- Automated change detection removes duplicate verification effort by flagging new or changed data from inbound sources.
- Automated checks identify errors with incoming data and generate queries without human intervention. Resolved queries are automatically recognized and closed.
- Clean patient tracker summarizes all patient data in one easy view, with drill-down to view patient and query data.
- Easily transform and export all study data in a single package, while standard export definitions simplify use for downstream systems.
- Veeva clinical query language (CQL) enables sophisticated listings with minimal effort.
Veeva eCOA
Veeva electronic Clinical Outcome Assessments (eCOA)
Captures questionnaire responses directly from clinical trial patients (ePRO), clinicians (eClinRO), or patient caregivers (eObsRO) using an app or webpage.
- eCOA library accelerates study design through reusable and validated eCOAs, sourced from both Veeva and sponsor libraries.
- Streamlined mid-study amendments and data change processes with complete audit trail and version control.
- On-demand data access across sites and study teams. No more waiting or data silos.
- Patient and Site notifications when ePROs require completion or are overdue.
- Optimized for BYOD including android, iOS, and web. Enhanced login options provide further flexibility, including username and password, PIN, or biometric authentication.
Why Veeva Clinical Data
Veeva Clinical Data brings innovation that redefines traditional processes. By automating the flow of data between operational and clinical systems, Veeva Clinical Data fuels the pace of today’s clinical trials. These result in tangible efficiency gains, including:
cycle times
and mid-study changes
cycle time
Learn more about how Veeva Clinical Data can streamline your data flow.