Features Brief
Veeva QMS Features Brief

Modernizing Quality Management
Greater regulatory scrutiny and the growth of global outsourcing makes quality management complex. Most companies use legacy systems that are not designed for collaborating with external partners.
Further complicating quality management, there are often separate solutions for managing controlled processes and regulated content. This gap between systems creates risk, prolongs cycle time, and magnifies manual overhead.
Veeva QMS is a cloud-based quality management solution that provides best practice processes for deviations, internal and external audits, complaints, lab investigations, change controls, CAPAs, supplier quality management, quality risk management, and proactive management initiatives. With a modern, easy-to-use web interface, Veeva QMS drives higher user adoption with minimal ongoing support.
Business Benefits
-
Enhance partner alignment.
Easily bring departments, sites, suppliers, contract manufacturers, contract test labs, and other partners into continuous quality improvement processes. -
Ensure greater compliance.
Ensure global compliance with seamlessly connected and controlled processes that are part of the Vault platform. -
Gain complete visibility.
Track quality processes proactively with the right level of visibility to support timely decisions and accelerate cycle times. -
Achieve faster time-to-value.
Leverage a ready-to-use, scalable application with built-in best practices and automated workflows to improve operational efficiency.
Features
-
Intelligent Automation
Maximize efficiency with embedded advanced technologies, such as recurrence check for quality events, duplicate check for complaints, automated email ingestion, and templatized change actions with sequencing capability. -
Delivered Quality Processes
Rapidly deploy standard quality processes for proactive management initiatives including deviations, internal and external audits, complaints, lab investigations, change control, quality risk management, supplier quality management, annual product quality review, and CAPA processes. -
Intuitive Interface
Drive high user adoption with an easy-to-use application. Providing a consumer experience streamlines tasks, and reduces training time, boosting productivity. -
Configurable Forms and Workflows
Using ‘point and click’ configuration, modify best practice workflows or create new processes. End users can quickly add tasks and link documents to a process. -
Robust Audit Trails
Annex 11 and 21 CFR Part 11 compliant audit trails t capture every event in a process, including execution of a signature, task creation and assignment, and more. -
Unified Risk Management
Easily incorporate risk management into existing quality processes across R&D and manufacturing. Effectively allocate resources with an enterprise-wide inventory of prioritized risks via Risk Builder. -
Quality-RIM Connection
Streamline change control and variation management by automating document and data sharing across quality and regulatory functions with the Veeva Connections. -
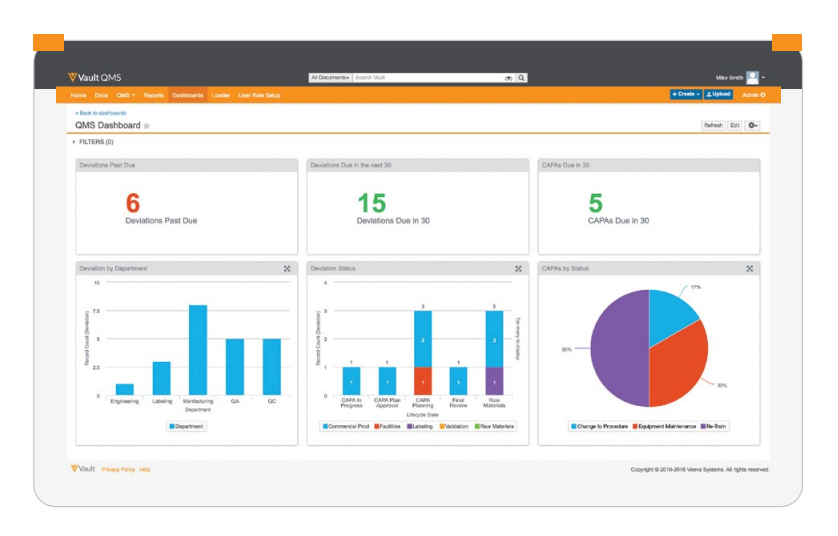
Reports and Dashboards
Gain actionable insights into quality events through self-serve reports showing information on different processes, including deviations, investigations, complaints, audits, CAPA actions, and more. -
Document Control
Automatically trigger a document change control for impacted SOPs, work instructions, or other content in Veeva QualityDocs, seamlessly connecting quality processes to critical documentation. -
Application Integration
The open, published Vault API easily allows integration with complementary applications, such as ERP, LIMS, MES, and CRM for streamlining processes across business systems. -
Supplier Access and Visibility
Easily provide access to suppliers and contract manufacturers, integrating partners into key processes for greater visibility and control. -
Mobile Solution
Use Veeva QMS application on any device, from anywhere. Easily check status, complete tasks, or fill out forms while away from your desk.
Veeva Quality Cloud
Veeva Quality Cloud accelerates the manufacturing of high-quality products to a greater number of patients. The cloud platform unifies applications, processes, and partners across content management, training, quality management systems (QMS), and QC lab solutions (LIMS).