Features Brief
Veeva SafetyDocs PVA Management Solution Brief
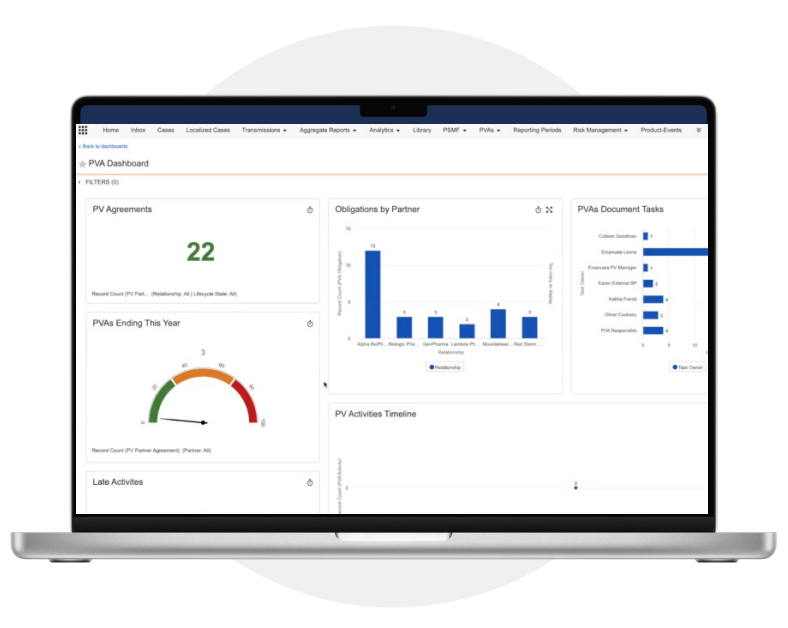
Increase efficiency and gain oversight of pharmacovigilance agreement obligations
Veeva SafetyDocs enables more efficient management of pharmacovigilance agreements (PVAs) and safety data exchange agreements (SDEAs) and provides oversight of PVA/SDEA obligations.
With secure access and customizable workflows, Veeva SafetyDocs makes it easier to collaborate with external partners, manage multiple PVAs/SDEAs globally, and track obligations for improved compliance.
Business Benefits
-
Improve partner adherence.
Gain oversight of partners and PVA obligations with task workflows and customizable reports and dashboards. Efficiently monitor activities for timely completion to reduce compliance risk. -
Efficient PVA lifecycle
management.
Centralize PVA documents and workflows in a single system to manage all of your PVAs and SDEAs from authoring through termination. -
Always use the right version.
Automated version control ensures the correct PVA version is always used and improves inspection readiness.
Features
-
Automated Obligation Scheduling
Automate recurring PVA activities to support real- time reporting and reduce manual effort. -
Reports and Dashboards
Track operational metrics to determine the status of PVA obligations, as well as oversight of partner activities and compliance. -
Real-time Document Distribution
Securely share documents with partners via email or a Vault workflow and track their activity, including receipt and acknowledgment. -
Configurable Workflows
Automatically generate tasks for external partners to ensure agreed-upon obligations are completed timely. -
Version Control
Automate versioning and easily compare documents to previous versions to see how the content was changed. -
User Access Management
Set up secure user access roles to define what content users are allowed to view or edit and what activities they can complete. -
Automated Notifications
Receive automatic alerts when a pharmacovigilance agreement is due to expire. -
Template Management
Create and store PVA templates to speed up the time to draft new pharmacovigilance agreements.
Watch demo to see how to improve oversight and tracking of PVAs.
