Blog
Breaking the Status Quo and Embracing the Redefinition of LIMS
Feb 13, 2025 | Ashley McMillan
Feb 13, 2025 | Ashley McMillan
With a founding mission to improve patients’ lives through high-quality biosimilars, one global biotech is reimagining how it works to prepare for exponential growth: from expanding its in-house capabilities to investing in state-of-the-art manufacturing facilities, and focusing on rigorous quality through vertical integration.
Yet, earlier this year, the company found itself at a crossroads in its lab journey. The laboratory information management system (LIMS) it had implemented in 2020 already seemed misaligned with its high-growth business model. It could either continue to navigate this system’s limitations and risk its business goals; or, it could switch to an alternative in the hope of truly modernizing.
Faced with a ticking clock, the company’s director of Veeva Platform decided to make the case to her team for the alternative. She describes the reaction after she proposed leaving behind the legacy LIMS solution during an IT workshop: “We were reviewing tactical and strategic ideas for next year, and I put up a sticky note saying ‘Veeva LIMS’ on the board under ‘strategic’. I will never forget the look I got across the room. We got a lot of ‘nos’ right there.”
Make do or modernize
Problems emerged soon after the biotech went live with its legacy LIMS in 2020 [Figure 1]. Following a lengthy implementation, the team soon realized that the system remained siloed from core processes. Instead of using its LIMS to improve productivity, the lab was forced to adapt its workflows to only what the technology permitted: for example, by adding documents to address the lack of a mechanism for capturing change analysis in master data.
Figure 1: Reaching a crossroads with legacy LIMS
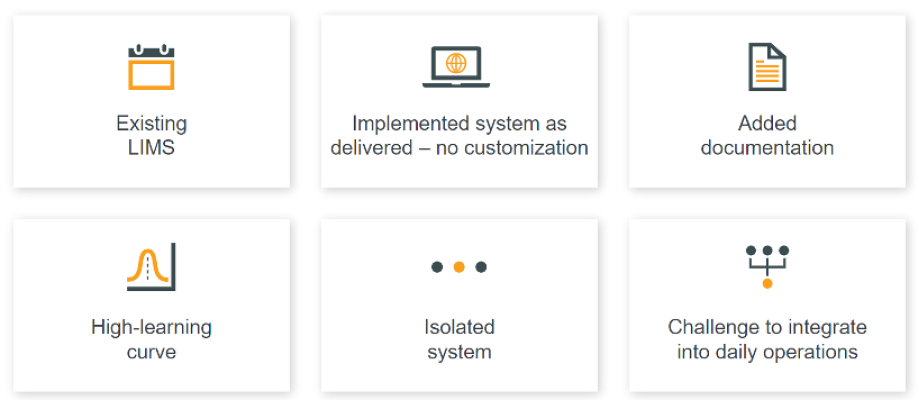
Source: Global biotech
Although the system was feature-rich, adoption soon stalled at just five percent usage of the available features. This was despite abundant training to help users master a steep learning curve. “But in practice, they couldn’t figure out how to use some of the features in the existing architecture such as the training module, or document management,” explains the director. As a result, manual processes persisted, introducing more paper and complexity. “We could only make incremental improvements toward automation.”
After receiving health authority (HA) approvals, the team realized continuing with its legacy LIMS would jeopardize its growth ambitions: the system couldn’t adequately support rapid product expansion or global launches. “The first signs of trouble were when we were trying to scale operations. We didn’t have the resources to manage changes in the system,” adds the director.
The company chose not to delay its transition and started evaluating alternative solutions that would help it scale during commercialization. “We wanted a modern system to streamline workflows, data control, and improve efficiency,” she says.
Making a head-and-heart decision
The company was already familiar with the Veeva ecosystem, through its Veeva Quality applications (QualityDocs, Training, and QMS) as well as clinical (Veeva eTMF) and regulatory (Veeva RIM). Knowing how easily these applications integrated into its processes (and could facilitate content and data sharing), the team felt confident Veeva LIMS would be a natural fit [Figure 2].
Figure 2: Choosing modernized QC

Source: Global biotech
Disrupting the status quo can trigger emotional resistance. Often, it is justified. The team had invested “blood, sweat, and tears” into implementing the old system yet faced the prospect of a new LIMS just three years later.
Instead of seeking harmony, the director recommends embracing team emotions and understanding the objections. Many end-users will have valid concerns that the business case should acknowledge and address. Inviting them to join demos puts the onus on your technology partner to prove their worth, as skeptics ask the toughest questions and will be among the most difficult to impress. After the second demo of Veeva LIMS, she remembers receiving a message from a key stakeholder saying, “This looks annoyingly good”, reinforcing confidence in the decision to switch.
Every business case will balance expected long-term value against cost considerations. However, it’s also worth calculating ROI within a shorter timeframe: for example, the additional capacity your business would gain within the next year if you switched versus the incremental returns of sticking with the existing system. The results may surprise you. The director points out, “We saw a higher projected ROI with Veeva LIMS than for the legacy system.”
A quick and smooth transition
Once the team evaluated Veeva LIMS more closely, a key differentiator was that it could incorporate the solution into its workflows without any disruption. Quality and regulatory professionals would keep accessing certificates of analysis (CoA) in the same way as before, ensuring operational continuity while the company upgraded. “Jaws were literally dropping as we saw how smooth the transition would be for something like CoA,” she remembers.
With commercialization just around the corner, a quick transition was critical. Veeva LIMS is designed specifically for pharmaceutical manufacturing, so the teams agreed to a streamlined seven-month implementation leveraging industry-standard best practices. This allowed the team to access additional capabilities quickly and support rapid user adoption. Veeva’s lifecycle development is “rapid and reassuring”, so the biotech knew it would benefit from new features further down the line without enduring onerous system upgrades.
To stay on schedule and realize these gains requires good visibility of technical capabilities and the expected implementation support. The team focused on new feature releases that would reduce data silos (such as batch release testing and stability study management). As customization is contrary to the company’s business model, Veeva LIMS is being configured to these specific lab needs, avoiding the prospect of a large IT footprint or heavy system maintenance.
Skilled resources will reduce their time and effort on manual data entry, forms, and other routine tasks to focus on more impactful activities. Automating workflows will reduce the risk of errors and increase right first time, as well as lower the total cost of ownership (TCO). As she notes, “We are investing in a solution that will support our long-term goals.”
Adopting a critical mindset
Now in the final stage of its implementation journey, the director credits clear and open communication within the extended project team for solid progress to date. “You should question what you did previously, whether you’re trying to keep things the same — and why,” she advises.
Breaking the status quo requires bravery, readiness to embrace other people’s emotions, and refusal to take ‘no’ for an answer. She concludes, “Our decision to move to Veeva LIMS was driven by practical and emotional factors. We’ve now made a choice that we think will position us for future success.”
Learn about the five common misconceptions preventing biopharma companies from modernizing QC.
