Veeva LIMS
Modernize Quality Control with a Unified Solution
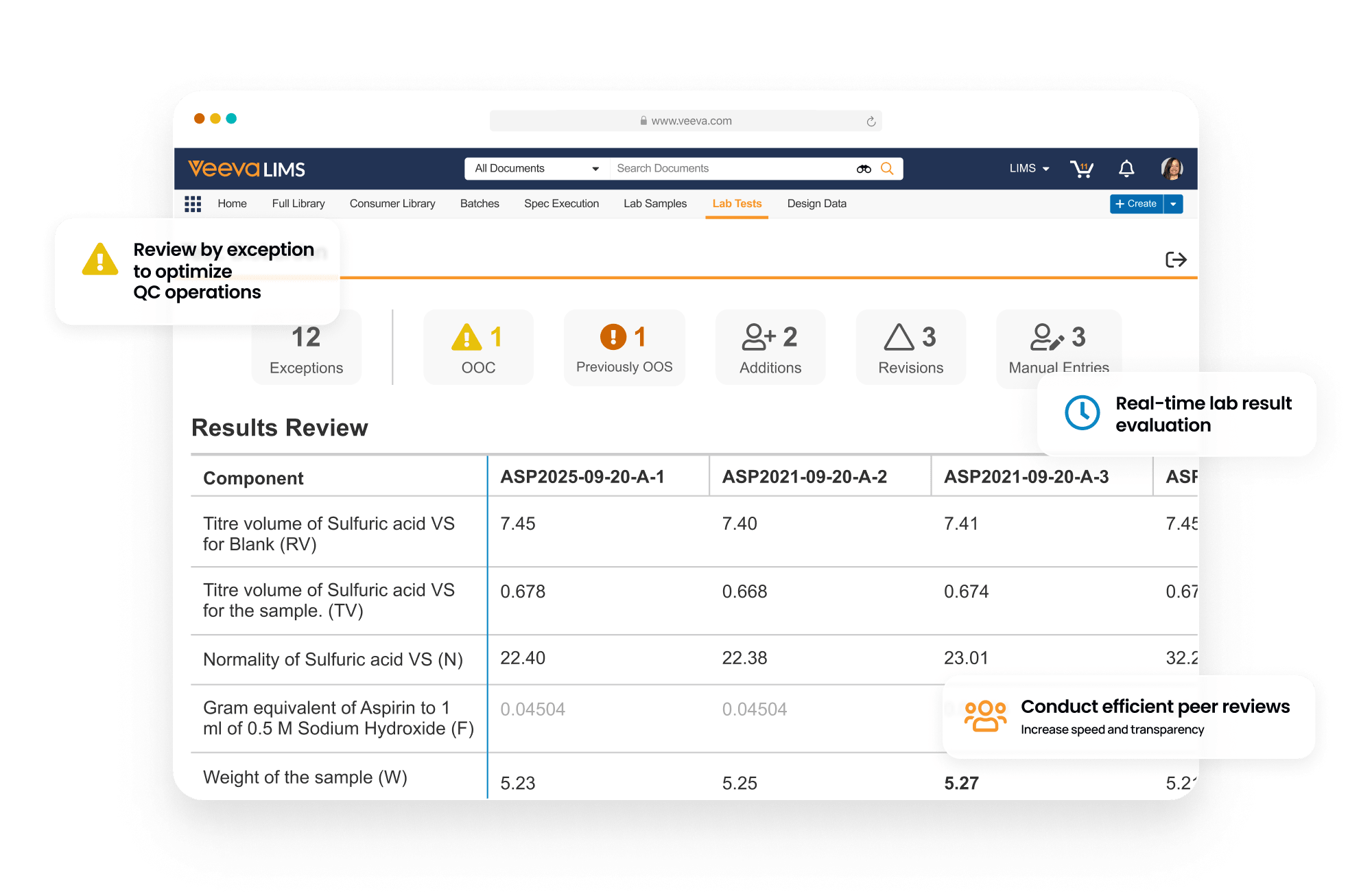
LIMS optimizes batch release testing, stability study management, and environmental monitoring for the quality control lab. It drives detailed sample management, digital test method execution, specification adherence, and review by exception to accelerate the release of product.
LIMS promotes compliance by verifying user qualifications from Training, displaying effective test method procedures from QualityDocs, and initiating quality events directly in QMS, ensuring proper resolution prior to batch disposition.
Announced 2021 Status Early Customers11-50
See how Veeva LIMS can modernize your QC operations
Overview
Modernize Quality Control with a Unified Solution
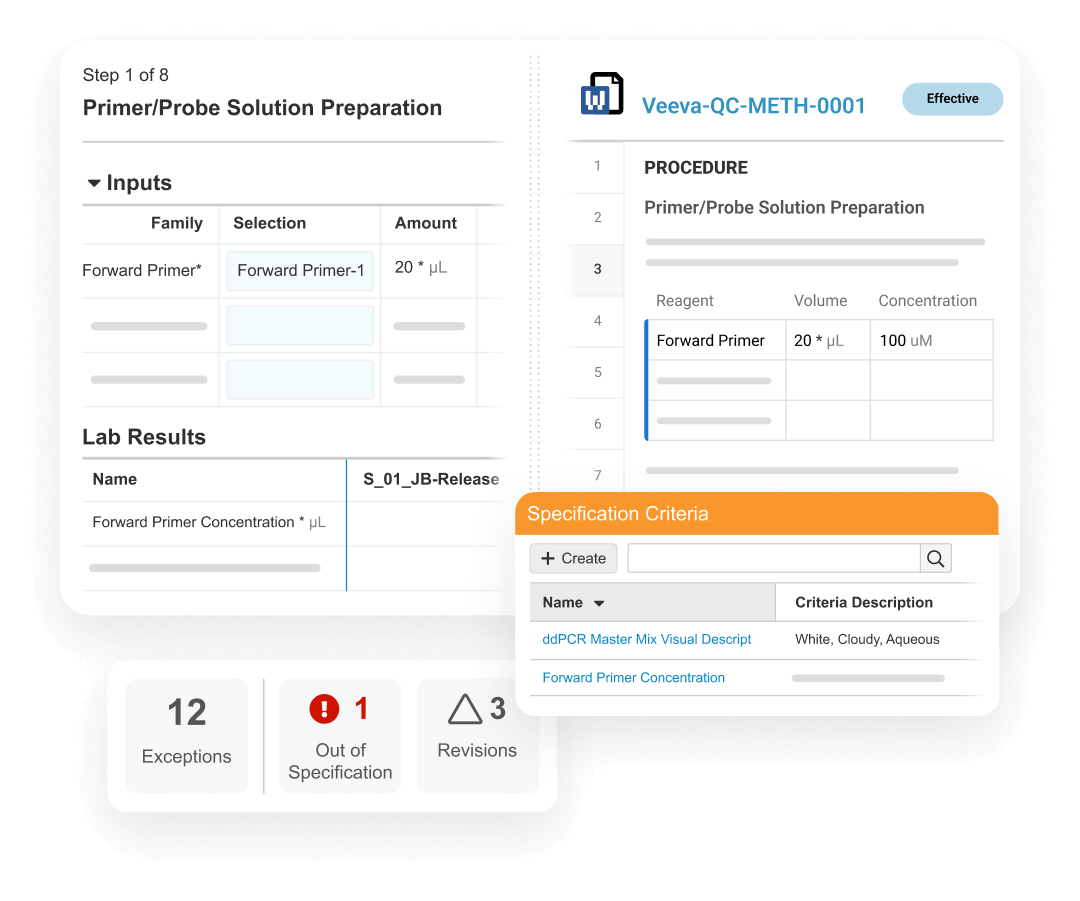
LIMS optimizes batch release testing, stability study management, and environmental monitoring for the quality control lab. It drives detailed sample management, digital test method execution, specification adherence, and review by exception to accelerate the release of product.
LIMS promotes compliance by verifying user qualifications from Training, displaying effective test method procedures from QualityDocs, and initiating quality events directly in QMS, ensuring proper resolution prior to batch disposition.
Why Veeva LIMS
Modern, unified quality control
Resources
Explore and Learn
Read White Paper
Learn, Confirm, Then Scale: A Leader's Guide to Transforming Quality Control

Read Features Brief
Veeva LIMS Features Brief

Watch Video
Why Veeva LIMS

Read Case Study
How Veeva LIMS is Helping Forge Biologics Scale QC for Rapid Growth

Read Case Study
Verve: Embracing Digital-First Quality

Read White Paper
Building the Business Case for LIMS

Read Blog
Modernizing LIMS with Embedded Test Method Execution

Read Blog
Breaking the Status Quo and Embracing the Redefinition of LIMS

Read Article
Entering the Modern Era for Data-driven Quality Control

