Veeva Submissions
Streamline Regulatory Submissions
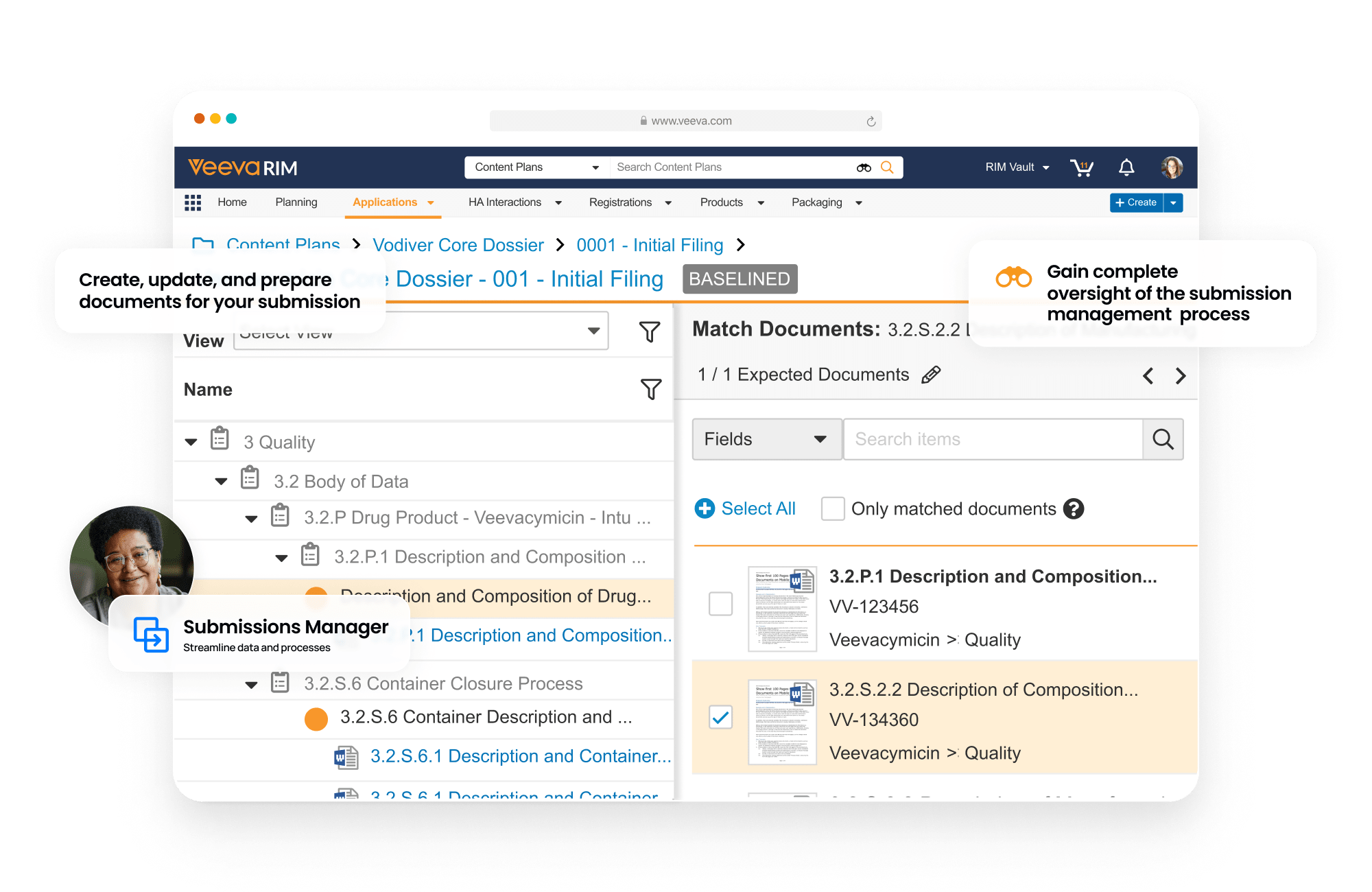
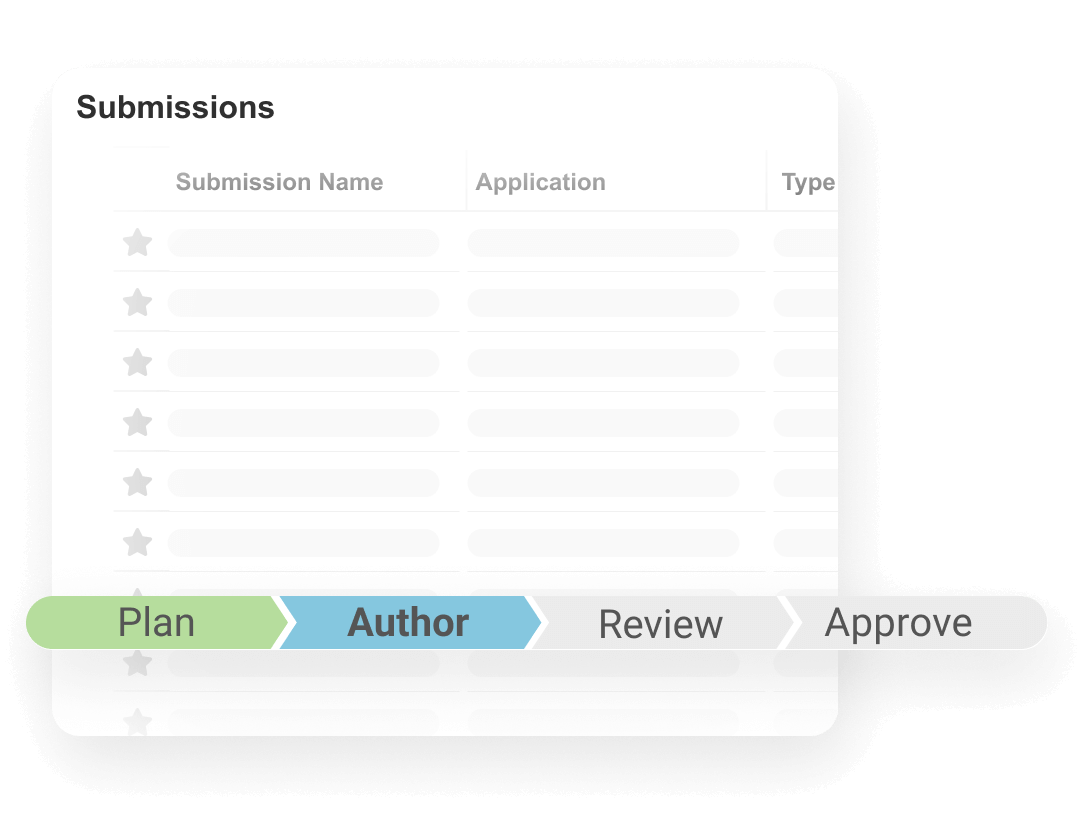
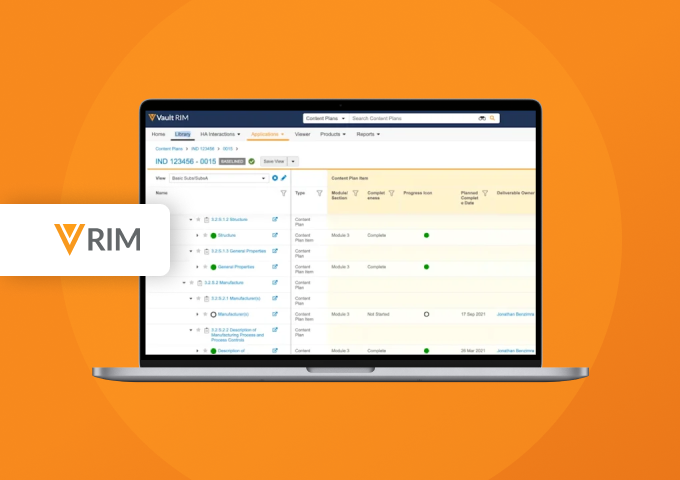
Submissions is a content management application used to plan, author, review, and approve regulatory documents. It provides full enterprise content management capabilities for creation, version control, approval, and real-time co-authoring for all submission-related documents. With content planning capabilities, users can build a submission outline and automatically match documents to the outline.
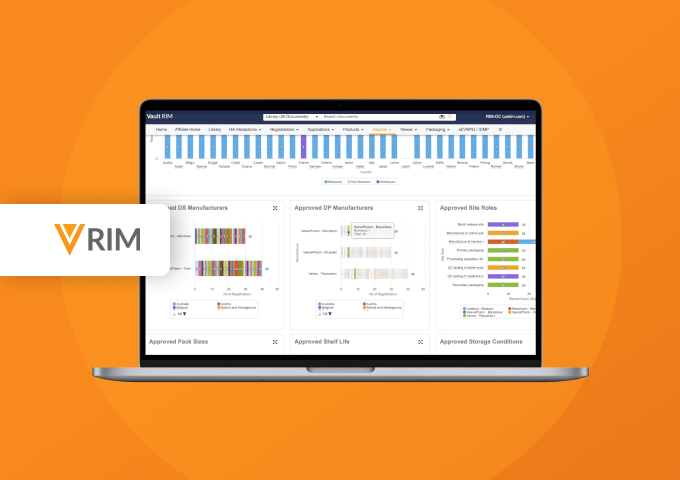
Clinical and non-clinical reports can be built and published using Report Level Content Plans. Dashboards and reports allow submission managers to track the status of each document in real-time.
Announced 2013 Status Very Mature Customers 100+
Discover how your regulatory team can become digitally mature
Overview
Streamline Regulatory Submissions
Submissions is a content management application used to plan, author, review, and approve regulatory documents. It provides full enterprise content management capabilities for creation, version control, approval, and real-time co-authoring for all submission-related documents. With content planning capabilities, users can build a submission outline and automatically match documents to the outline.
Clinical and non-clinical reports can be built and published using Report Level Content Plans. Dashboards and reports allow submission managers to track the status of each document in real-time.
Veeva AI for Regulatory
Health Authority Interactions Agent
Automates HA interactions for faster simultaneous approvals globally.
Application Assistant Agent
Conversational insights into regulatory activities with clear and consistent narratives.
Impact
Exceed the likely outcome
15
of the top 20 companies use Veeva RIM
88
IT systems consolidated into one
90%
reduction in written standards
Why Veeva Submissions
Single authoritative source
Customer Success
Veeva RIM is trusted by 400+ top
and emerging biopharmas




















Resources
Explore and Learn
Read Features Brief
Veeva Submissions Features Brief
Watch Demo
End-to-end Veeva RIM Demo
Read Best Practices
Guide to Successful RIM Implementation
Read Features Brief
Submissions Content Planning Features Brief

Watch Video
Submissions Content Planning Demo Video
Read More
Three Key Insights to Get the Most Out of Submission Content Plans

