Article
Connecting Med Info, Safety, and Quality to Improve Operational Efficiency
Modern drug innovation has changed the treatment paradigm. Increasingly challenged to stay current on the latest developments and scientific literature, healthcare providers (HCPs) are demanding deeper scientific exchange to support their clinical decision-making. They’ve come to rely more heavily on medical information as a trusted source of product education and primary touchpoint for reporting potential adverse events (AEs) and product quality complaints (PQCs). Biopharma companies have sought to create more internal synergies between medical information, safety, and quality teams, but existing frameworks have fallen short of overcoming the high degree of manual effort required to transfer and reconcile data compliantly.
We recently sat down with a global medical operations lead at a top ten biopharma company to discuss how his team overcame institutional challenges to connect this critical triangle and how organizations can get started driving transformation of their own.
The impetus for transformation
In a recent survey of US physicians, 60 percent of respondents said the integration of biopharma company interactions across channels was crucial to their experience.
Companies are adapting their engagement models to meet these evolving preferences across a wider mix of channels. To do this effectively requires orchestration behind the scenes. For this company, an area ripe for improvement was at the intersection of medical information, safety, and quality.
The executive explains, “Our company cares about efficiency, productivity, and driving commonality across the enterprise. It’s not as flashy but the results are tangible both in time and potentially money saved down the road.
The intersection of medical information, safety, and quality
Organizations typically have procedures that define the compliant way to collect, process, and report AEs and PQCs. However, lack of clarity on the processes shared between teams creates confusion around who owns what.
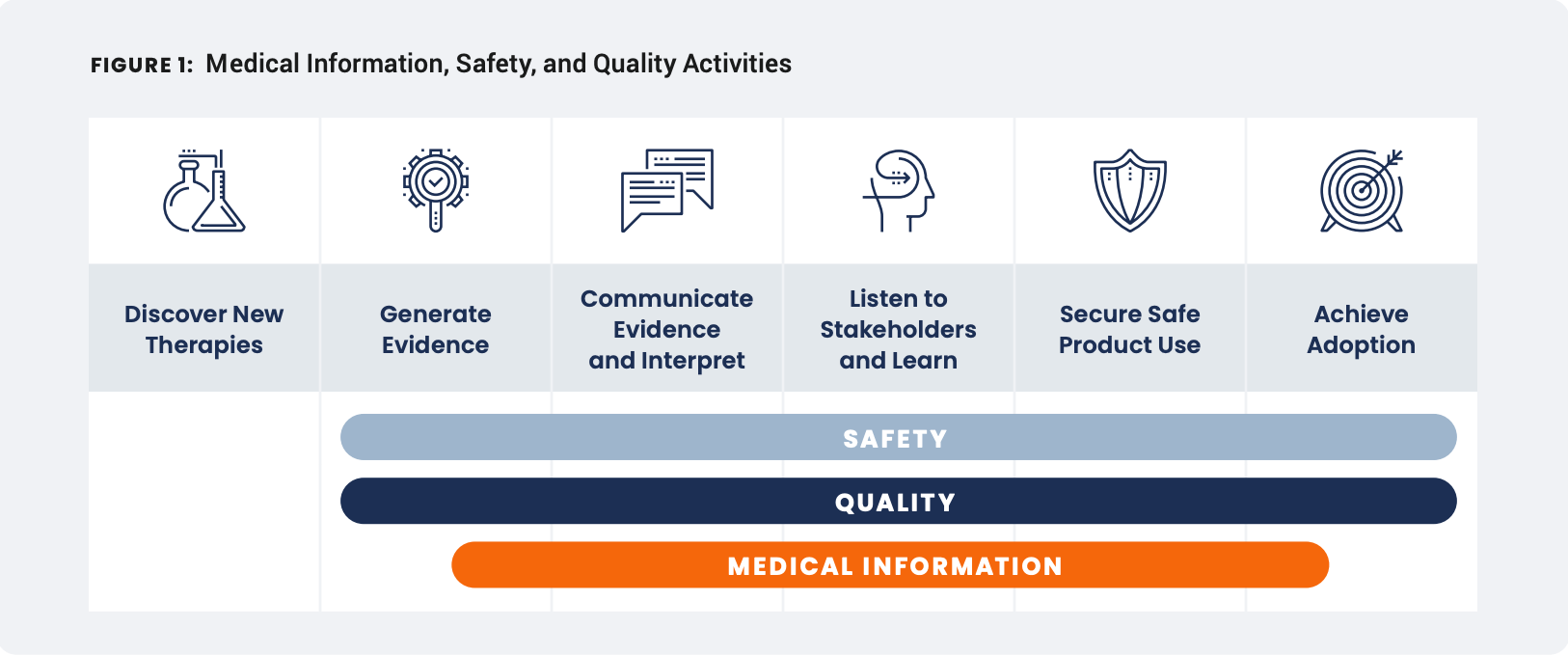
When data and technology platforms are disconnected across functions, the time spent reconciling these tasks is critical time lost, not just for HCPs and patients but for teams managing high case volumes across products and geographies.
The flip side of that coin is compliance. Siloed execution carries serious risks. As the executive points out, “If we make a mistake in any of these realms, it’s significant.
It can have regulatory implications. That manual effort is fraught with risk.” The opportunity to simplify and streamline processes across teams is clear but new advancements in connected technology solutions are providing organizations the means to act.
Breaking down the silos
On a path to replace their medical information intake system with Veeva Vault MedInquiry, the global company saw a larger opportunity to establish an intake model in partnership with safety and quality. As the executive puts it, “When we’re talking multinational, multi-therapeutic areas, complexity of country, region, different time zones – having a standard intake model just makes sense.”
The company took advantage of Veeva’s native connectivity across products to build a new coordinated approach between teams. Creating this alignment upfront has numerous downstream benefits. “It’s adhering to our same policy standards, but it’s more efficient,” the executive explains. “It’s keeping our external HCPs in mind as it pertains to the crosstalk that has to happen in reconciliation.”
Leveraging Veeva Connections, the company has been able to:
- Standardize cross-team processes across different Vaults
- Harmonize data management driving commonality between functions
- Automate manual work without the burden of integration and maintenance
- Mitigate risk by providing full traceability from one system to another
- Simplify the IT landscape by sharing a single core platform and common release cycle
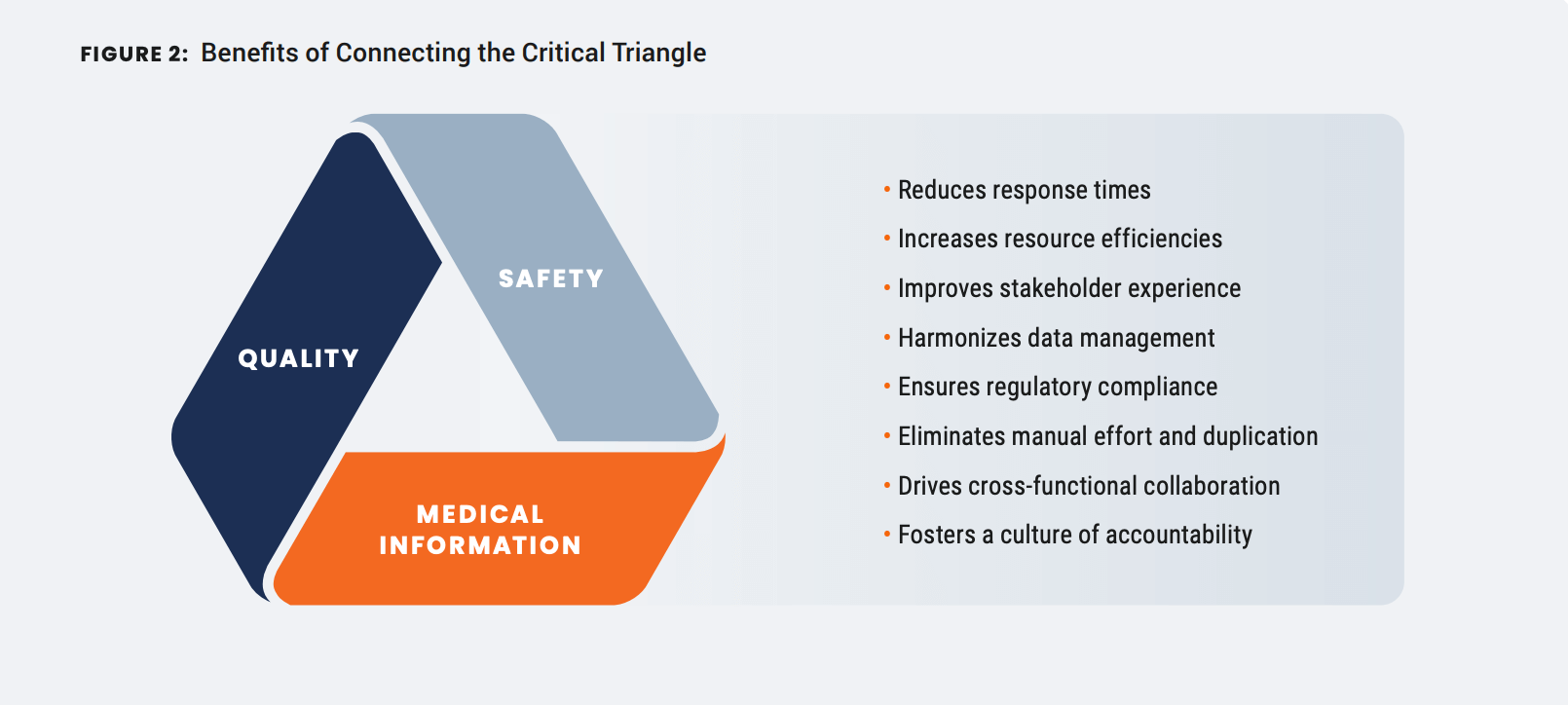
Three pillars of unification
Here’s how to think about your foundational components when looking to unify medical information, safety, and quality:
Baseline current capabilities
Evaluate existing capabilities from intake and triage to reporting and reconciliation. What are the strengths and weaknesses? Document current pain points and their root causes and then identify the areas needing improvement, whether that includes gaps to be filled, or inefficiencies that can be addressed through automation.
Define the north star for collaboration
This is your destination. If there is an opportunity for stronger connection across functions, define why this is important and what you are trying to achieve. Your north star should clearly articulate the case for action, detailing why addressing this opportunity is crucial for the business and outlining the potential risks of inaction.
Map the future state
Identify your target capabilities and design the roadmap to meet them. Teams should collectively agree on desired outcomes at each level, with clearly defined accountabilities and success measures. Consider this multi-phase framework for action.
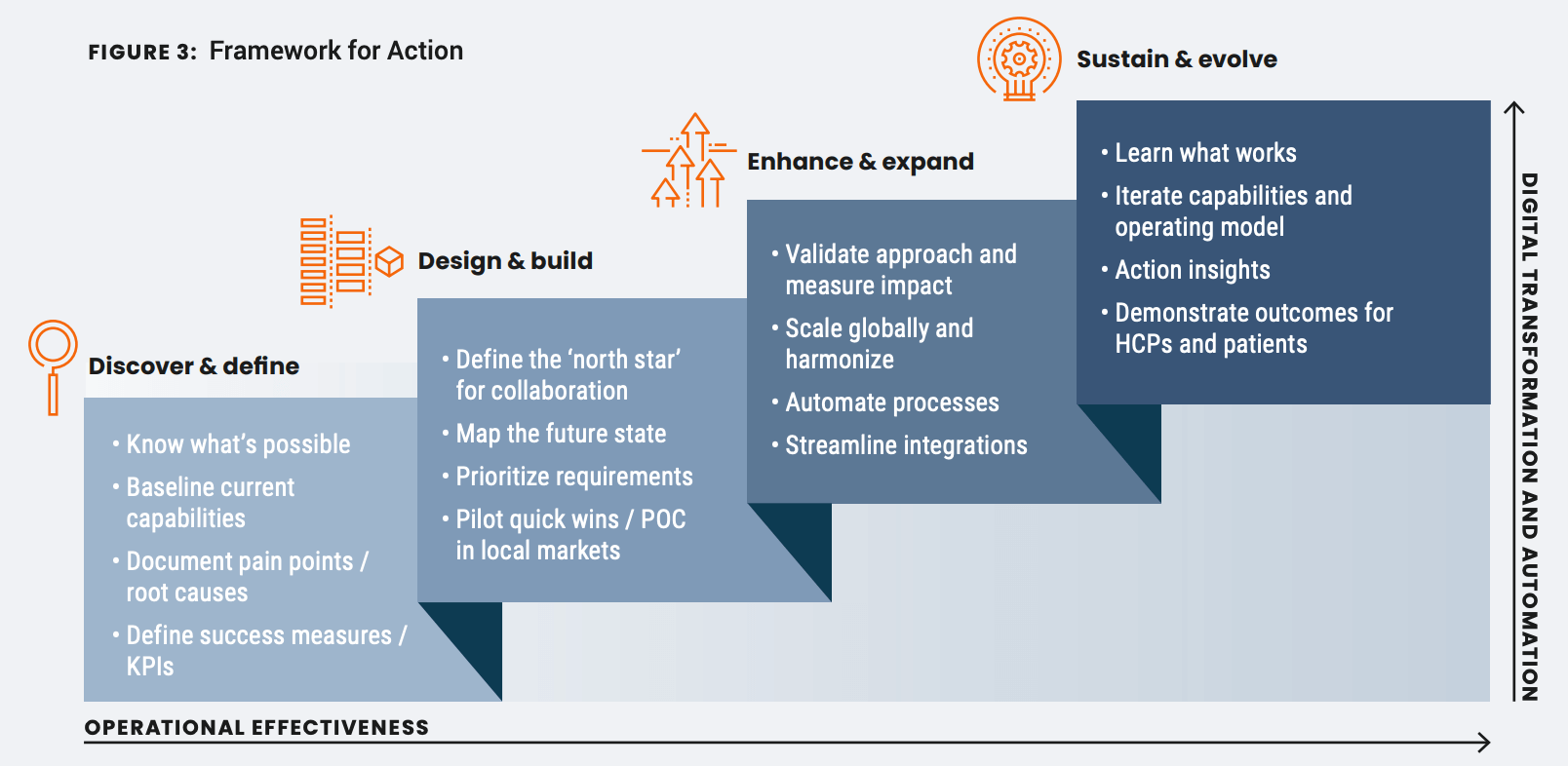
Remember, transformation is an ongoing process. It doesn’t have to be the full suite of capabilities from day one. You can be practical about these improvements occurring over time, finding pockets of value along the way.
To learn more about improving collaboration between medical information, safety, and quality, get in touch with Veeva Business Consulting.
AUTHORS
Allie Reynolds Senior Director, Vault Medical Strategy
Danielle Pagnan Engagement Manager, Medical Business Consulting danielle.pagnan@veeva.com
Mary Verdin Alcazar Principal Business Consultant, R&D Mary.alcazar@veeva.com
