eBook
Creating Effective Biopharma Content: An Agency Perspective
Does your life sciences company have a comprehensive picture of how field reps use the content you create? Can you easily measure the content’s effectiveness in better engaging healthcare professionals (HCPs)?
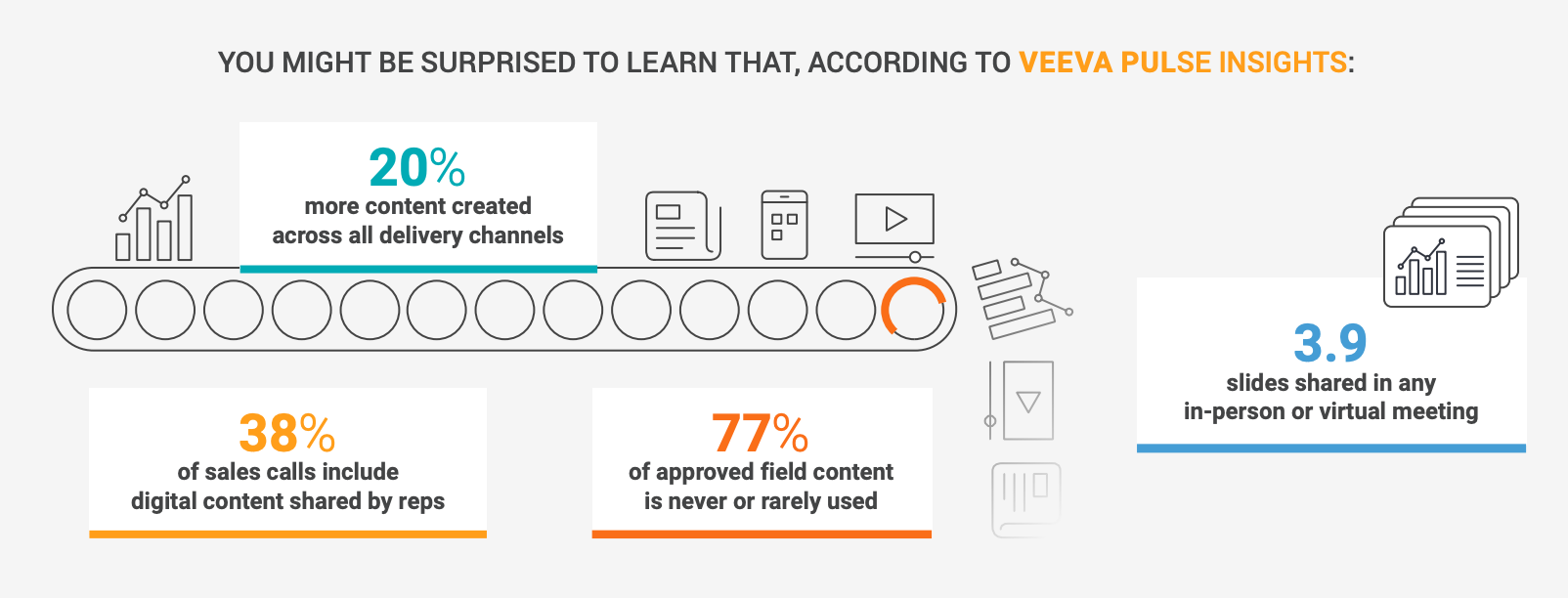
These statistics highlight opportunities to improve the content creation process. As the demand for relevant content increases, constraints on time, budget, and personnel — coupled with aggressive workloads and delivery schedules — can leave less time for collecting and assessing the data you need to ensure your content’s effectiveness. Failing to collect and heed content usage data creates a gap between the materials you create and the assets your field teams need.
Nonetheless, the content that field teams use still carries significant promotional impact. For example, patient starts are 2.5 times more likely to occur when field reps share content with HCPs in meetings, as compared with instances where no materials are shared.
How can brand and marketing teams create impactful, relevant content efficiently so they spend precious resources on only the most effective content? The answer lies in modular content — pre-approved components for use across individual content pieces, delivery channels, and regions. The right agency partner can help you collect the right data, implement the most efficient content creation process, and make better content marketing decisions overall.
The benefits of modular content creation
Modular content is tailor-made for collaborative global and local brand management. Creating modular content for use in your content marketing materials provides several benefits:
- Content reuse for personalization and localization: Pre-approved content modules allow you to repurpose existing content, reducing the cost and effort associated with creating new content. For example, you’re able to repurpose existing modules to create customized content for a meeting with a specific healthcare professional (HCP) or overhaul a campaign for a different market.
- Faster medical, legal, and regulatory (MLR) approvals: Approved content modules can be used in new pieces without resubmitting them for MLR approval, placing them in the hands of field teams faster.
- Comprehensive insights: You can track the use of content modules across many pieces of content, cross-referencing the data with the effectiveness statistics of individual content pieces. Module tracking yields more comprehensive and granular data on content performance.
Adam Hirsch is executive vice president of innovation and transformation at Real Chemistry, which drives the commercial success of healthcare companies by creating AI-powered, idea-driven healthcare experiences with the customer at the center. He sees the use of modular content as a tool to enable the creation of tailored and compliant content faster and at scale, improving its usefulness to your field teams. Hirsch emphasizes just how effective modular content is with regard to reuse and personalization. “The more you personalize your content, the better. There’s a misconception that personalized content means completely new content. But reuse is critical.”
Digital asset management
The benefits of modular content begin with the ability to effectively organize it. Content modules such as master slide decks, product shots, global and localized language for product descriptions and usage, composites, claims, and references can be stored in digital asset management (DAM) — a library or “vault” for approved content. DAM is a single source of truth, so you can easily manage the accuracy and compliance of the content as you update it. Content and field teams can easily search those components and use each module to assemble and personalize existing content. This can be done on the fly for specific meetings, presentations, or new distribution channels.
“Multi-channel content is too often built separately and, therefore, inefficiently,” says Christen Nyarady, executive vice president of delivery optimization at Omnicom Health Group. “Once you have accurate, approved content cataloged properly using digital asset management, you can more efficiently personalize content for different delivery channels.”
"Once you have accurate, approved content cataloged properly using digital asset management, you can more efficiently personalize content for different delivery channels." - Christen Nyarady, EVP, Delivery Optimization, Omnicom Health Group
What goes into creating effective content?
Best practices for content marketing take on added importance for biopharma content teams that build content for highly differentiated audiences that face MLR reviews. Those best practices include:
- An understanding of the content journey: Beyond accommodating various baseline personas, a field rep’s presentation may vary by HCP or medical specialty, knowledge level about the therapy, and the target’s location. A granular understanding of who the prospect is, what they already know about your company, what therapy they are most interested in learning about or discussing, and the format to which they’re most receptive are keys to moving the sales process forward. The more personalized the content, the more likely a prospect is to read, view, or listen to it. A library of pre-approved modular content can speed up the work a rep undertakes to personalize content.
- Strict compliance adherence: Any organization’s marketing content must comply with certain corporate standards. This need is complicated by numerous factors, among them: input and approvals from stakeholders organization-wide, integration of an outside content agency into the workflow, and the need to accommodate localized versions of global content. Of overriding importance — and of unique concern in biopharma — are MLR reviews of promotional materials. These reviews can become stumbling blocks for efficient content creation, but involving your MLR team early can improve the content creation and approval process.
- A standardized approval process: From MLR and conceptual approvals to reviews by brand, product, and field teams, it’s imperative for your whole team, including your agency, to follow a standardized, well-documented process to achieve sign-off on a biopharma marketing asset. Adherence to the process will accelerate production while keeping the piece on-brand and on-message, and prevent it from running afoul of regulatory requirements. Similarly, the process for making changes to content must be standardized to ensure that no unvetted language finds its way into the content. This could happen, for example, if your team makes updates to boost a piece’s overall effectiveness based on engagement data.
- Data collection: It’s best practice to use quantitative data from your DAM — not guesswork — to determine what content to create. A DAM should provide metrics that identify what content is being used, what isn’t, and where the gaps are in terms of topics that field reps use to start personalized conversations with HCPs. Vikki Ward is European chief of operations for Havas Creative Group and Havas Health & You, a global creative agency that works regularly with biopharma companies. She advises supplementing data from your DAM with qualitative feedback from field teams. “The way to find out why your content isn’t being used is to ask the people who are — or aren’t — using it: your field force. Field reps know whether the content is moving an HCP through an adoption ladder,” she says.
Working with your agency to build successful modular content
When creating and managing modular content for your field teams, tap into the following leading practices:
Build partnerships and foster trust to enable collaboration
Partnership with your content agency should extend beyond your internal content team. Ensuring your agency can work comfortably with every content stakeholder will build trust, encourage collaboration, improve efficacy, and align all stakeholders on challenges, goals, and measures for success.
Jennifer Ma is executive vice president and group director of operations and production at AREA 23, McCann Health New York, and AREA 23 on Hudson. She stresses the importance of collaborating with your agency: “You can build a giant tactical ecosystem, but if no one‘s on board with it, you won’t be successful. Your entire team — MLR, brand marketers, conceptual reviewers, field reps, as well as your agency — has to be on the same page.”
“Get everyone around a virtual table and discuss everyone’s feedback rather than circulating a draft,” advises Ward. “This will not only save time and improve alignment, it will foster teambuilding for the future.”
Make MLR your closest ally
Your MLR team should be your advocate, not your antagonist. Building relationships with MLR early on is essential. “The sooner you explain your strategy to MLR, the earlier they can voice their concerns,” says Hirsch, adding, “That can lead to great discussions, true collaboration, faster approvals, and fewer late-stage rejections.” It also builds trust among the entire team for future collaborations.
Be sure to include your agency in your partnership with your MLR team. “This aligns everyone on the content or tactical plan and builds a stronger relationship with, and confidence in, the agency,” notes Nyarady.
Your field force is an important source for content evaluation
How do you measure the effectiveness of your content campaigns? No single set of KPIs is right for measuring the success of every piece of content, and valuable data can and should be quantitative and qualitative in nature.
“The most important metrics are the amount of time someone spends looking at the content and how often our field teams use it,” explains Ma. Because your field reps are the ones using your content, getting their feedback is essential.

Field reps can provide you with a real-world understanding of whether your content is easy to find, use, and customize. They can tell you whether it’s resonating with its intended audience and what follow-up content will be of most use to them — from an email to a data portal to a PDF of a presentation deck. This input will help you improve the content that’s used most often, discard the content that goes unused, and spend resources creating new content that is most needed.
Review the data regularly and, most importantly, share it with your agency. “Your agency needs that data to be able to do the best job for you,” Ward says.
Localize your content and then encourage its adoption
Localization of content is another area that benefits from the use of modular content, but the needs of each local market where the content will be used must be considered during the conception and ideation phases of content production. You can encourage the use of content by the local field team by including them in the content creation process and then demonstrating that a piece of content has been conceived for, tested in, and assessed by a local market. A truly global co-creation team can create individualized, local content that’s in line with the global messaging and goals for the content.
Conclusion
Biopharma companies are publishing across more channels and localizing content for more markets. To meet the demand among HCPs for timely and relevant information, companies need to efficiently create content that the field team can and will use. Partnering with content agencies can help streamline operations while improving content quality and effectiveness. A modular content creation strategy can accelerate MLR review, improve adoption among field reps, and make personalization and localization easier while providing HCPs with information that moves them along your customer journey.
Modular content is the most efficient way to store, share, reuse, and update your content. It can also maintain its compliance and measure its usage. Veeva PromoMats facilitates the use of modular content, increasing speed to market by more than 50%, reducing content creation costs by 20%, and enabling 75% of all content to be reviewed in a single cycle, on average.
Veeva has a dedicated team and program to support content partner agencies that are trained to assist commercial and medical customers across the entire content journey. Learn more about agency best practices.
