eBook
Making Inbound Channels a Reality:
Insights From Commercial Leaders
A little over a decade ago, the life sciences commercial organization experienced one of its most significant changes: the advent of mobile devices for detailing. The industry took on this challenge together, devising different forms of training, change management methods, and new ways of working around these devices. Today, field teams reap the benefits of efficiency and better HCP insights thanks to our collective efforts.
The industry is going through a similar transformation now with the consumerization of healthcare. As other sectors optimize for metrics like response time, time to resolution, and resolution rate, the biopharma industry is being pushed to take the HCP customer experience to the next level.
Many companies have been experimenting with inbound communications and activating channels where HCPs can proactively contact reps whenever they need help. It's a paradigm shift away from the traditional outbound model, but based on what we've heard from commercial leaders, these new channels have been crucial to helping reps achieve a higher standard of HCP service.
Let's look at some of these perspectives shared by commercial leaders across the industry.
Social Norms Are Overriding Biopharma's Rules of Engagement
The industry has long been apprehensive about sanctioning inbound channels, primarily instant messaging, because of the difficulty in monitoring them and enforcing compliance oversight. However, HCPs and reps seem to be using these channels anyway, and this is a factor that's been pushing many commercial leaders to take a second look at enabling an official inbound channel for their field teams.
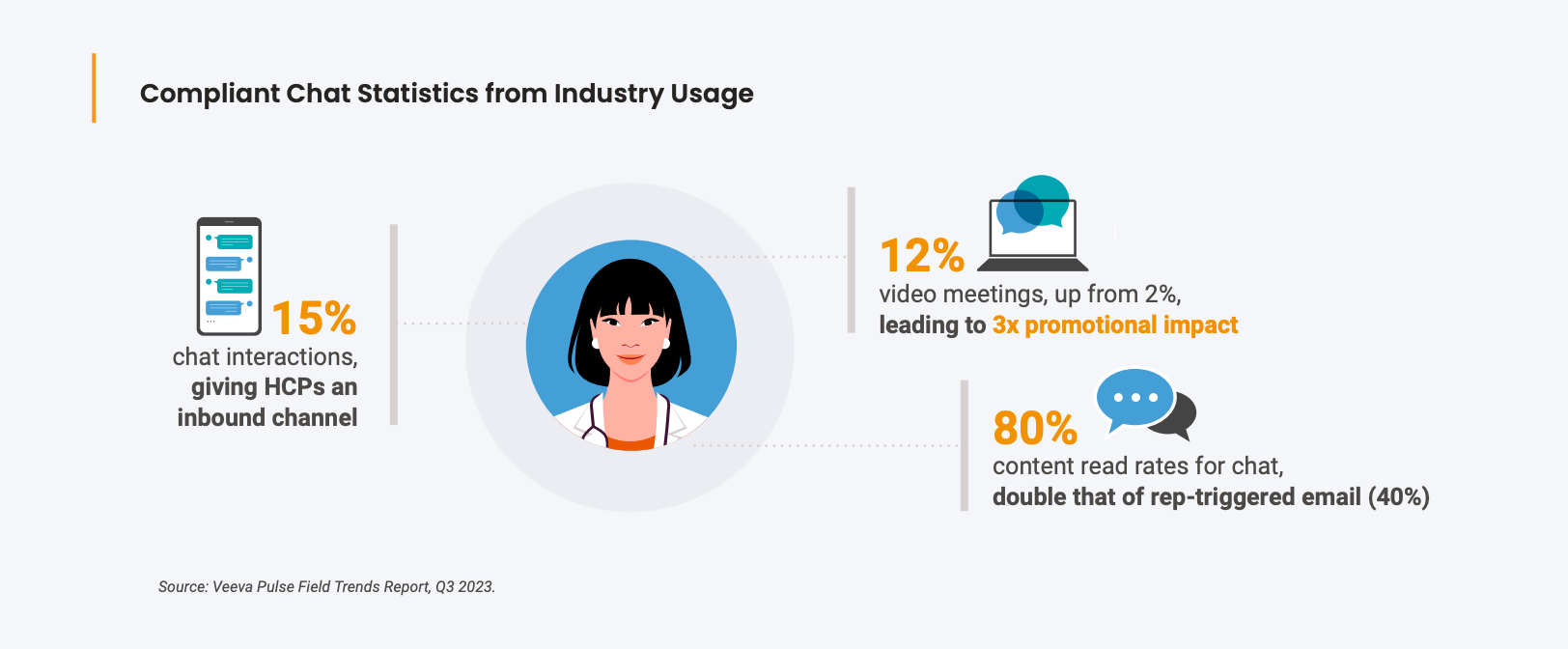
Commercial leaders across the board saw an increase in HCPs asking field reps for text messaging—or even texting reps outright. HCPs on close terms with their reps often texted reps when they needed help or materials, such as a specific study or a refill on samples. Though these communications frequently went against compliance guidelines, the leaders agreed that communications over text were widely used as an "under the table" channel, primarily due to HCP demand.
Many leaders viewed this change as a natural evolution of how people spoke with each other. Today's standard for customer service has shortened the response time to a customer query to just minutes. If HCPs can't receive service from a rep when they need it, it may leave the HCP with a negative impression of the brand and make the HCP less likely to prescribe. Conversely, if a brand could deliver service in more places and at more times for the HCP, it could make an HCP more likely to prescribe the product to their patients. By reaching out to the field teams for support, HCPs also open up more opportunities for conversations that establish rapport between them and their field rep.
Overall, the leaders agreed that inbound channels were necessary, but they still had challenges getting other stakeholders on board.
"Face-to-face access was difficult, and we were trying to find new ways to engage our customers. The field teams asked me for text messaging—they wanted a different way to interact with customers." Field force operations director at a large biopharma
"I've been fortunate enough to hear doctors say one-on-one, 'Can you just text me that?' We know that texting is a valuable channel. I think it's just something from an evolutionary standpoint that organizations need to be comfortable with." Commercial center of excellence lead at a mid- sized biopharma
Getting Compliance Onboard
Compliance was a key stakeholder for commercial leaders when evaluating inbound channels. While many compliance teams were concerned about taking responsibility for an officially sanctioned messaging channel, commercial leaders shared that when compliant chat is introduced correctly, compliance teams can grow quite supportive. From the experiences of these leaders, three best practices emerged for presenting the idea of a compliant chat channel to compliance teams.
1 Involve compliance early in the project
Some leaders have already successfully implemented an inbound channel, like Veeva Vault CRM Engage. A key learning they have is to bring compliance in at the very beginning of the process when different solutions are still being evaluated. Doing so gives compliance control over the decision-making process and helps them get more accustomed to the idea of HCPs reaching out.
Consulting compliance early on gives them a better understanding of each solution's tracking, monitoring, and prevention features. They can provide input into selecting the solution with the best capabilities to safeguard the company. Later, they can continue to provide feedback during the implementation and ensure that the configuration and user training are optimized for the latest regulatory requirements.
2 Work through risk scenarios
Another approach that's worked well with compliance teams is walking them through each risk scenario and devising protocols to handle each situation together. This process allows them to realistically evaluate the risk posed by the inbound channel and formulate solutions to problems that may arise after the channel is activated.
Response measures to compliance violations aren't always solely handled by the compliance team; they often involve IT, commercial operations, legal, and the field. By running through risk scenarios early on, compliance can establish procedures for how the cross-team should coordinate in each scenario. This way, if a violation happens when using compliant chat, each team already knows what to do to manage the situation promptly.
3 Show qualitative and quantitative benefits
Some commercial leaders also pointed out that compliance teams are often risk- averse and must see a solution's benefits before taking additional risks. In these cases, it can help to find a field leader who recognizes the value of compliant chat for their representatives. This field leader can share concrete use cases with compliance to show how having compliant chat can serve HCPs better and help representatives build stronger HCP relationships.
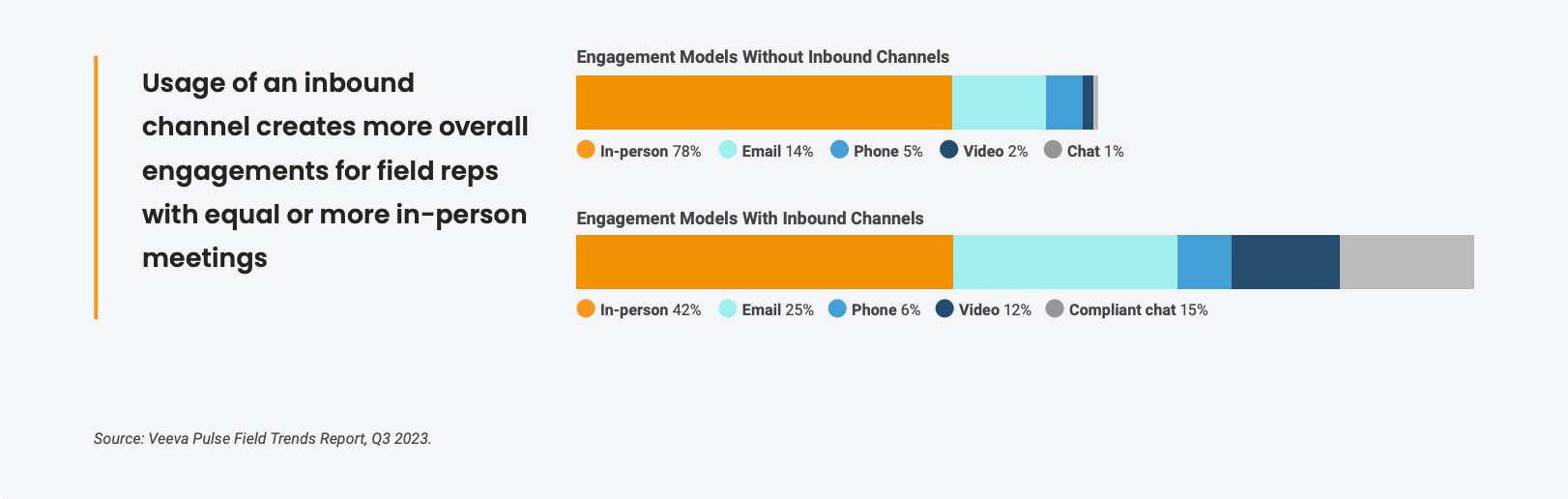
Performance metrics may also help back up the field leader's case. Veeva Pulse data has shown that opening a compliant chat channel doubles representatives' digital interactions while maintaining the same number of face-to-face calls. The same study also indicates that compliant chat has an 80% read rate, higher than any other channel.
"Bring your compliance officers along for the ride. We need to have them in these discussions with us, and we need to give and take. We no longer live in an environment where our customers won't allow us to engage in a more consumer way." Field force operations director at a large biopharma
"We had those conversations early on with compliance. We walked through all the use cases and the scenarios. If the worst-case scenarios happen, what do we do about them?" Field force platforms director at a large biopharma
Motivating the Field Force
Many commercial leaders mentioned the reluctance of field leaders to use inbound channels because they believe that the digital interactions in the channel may replace face-to-face communication. Others felt hesitancy from field teams about changing their working methods.
However, for the leaders who have made it past these conversations, these were simple objections to navigate once they gave the field the proper context. They shared the following learnings about managing this change.
1 Presenting inbound as a complement to face-to-face
Some field teams may feel threatened by a digital channel because they believe that digital channels would replace in-person HCP interactions—and eventually the reps themselves. The leaders who got their reps to buy into compliant chat handled this objection by giving specific use cases for how the reps were supposed to use this channel and how these use cases were not being addressed today.
For instance, using compliant chat, the field could coordinate with an HCP's office staff before lunch and learns, send along studies or materials after the HCP requests them, and take questions from HCPs about prescribing assistance or managed care. It was also essential to show the field the difficulty of addressing these use cases with the traditional face-to-face model and how addressing each case through compliant chat enhances the in-person relationship.
It is also helpful to emphasize how the rep is still the "quarterback" of the HCP relationship. They still control how and when they speak to HCPs and what messaging they present at certain times. The only difference is that the HCP can now contact the rep when they need help.
2 Finding compliant chat superusers to lead the way
Another strategy was to find early adopters of compliant chat in the field who could help motivate their teammates. Commercial leaders would identify field leaders who saw the potential of compliant chat and representatives who picked up the capability quickly to give the field force support at all levels.
The field leaders scheduled routine meetings and kept the entire field force accountable for using the compliant chat channel. During these meetings, the field representatives would share their experiences with compliant chat and develop best practices with each other. When the field was out seeing doctors, early adopter reps for each region supported their colleagues in case they encountered any technical difficulties with compliant chat.
"You want someone from the field with that credibility to talk to what they're facing. This person needs to know how this channel can help them with their objectives and help drive change within the organization." Field force platforms director at a large biopharma
3 Monitoring metrics and gamifying adoption
Many biopharmas that have successfully implemented compliant chat said they also took a data-centric approach to push adoption. They identified key adoption metrics at the beginning of the project—such as the number of HCP connections made, the number of chat conversations initiated, or the number of chats sent per connection—and measured these metrics periodically. If certain regions or reps seemed to be falling behind, home office could figure out the blockers and help them resolve any issues.
Taking these measurements also allowed home office to gamify compliant chat adoption, where reps could compete against each other to get the highest number of connections or chats. These metrics could also be considered during the incentive compensation process to reward reps for using the compliant channel to complement in-person touchpoints.
"Compliant chat is an opportunity for continuous engagement. And the data bears this out: Our in-person has pretty much remained the same after implementation, but the increase in other channel engagements has been impactful in that these are touchpoints that we might've otherwise not had." Field force operations director at a large biopharma
How the Field Benefits from Inbound
In addition to commercial operations leaders, we also chatted with field leaders to get their take on inbound channels. Field leaders found them helpful for expanding HCP access and building deeper relationships of trust with HCPs.
A sales director said her reps have opened up new opportunities for HCP conversations using inbound—specifically Engage. She gave the example of a rep who had hosted an educational event at a nephrologist's office. During the event, a cardiologist she was working with sent a message asking for assistance procuring a drug. After the rep finished the event, she borrowed an empty room in the nephrologist's office to have an Engage Meeting with the cardiologist. They got the pharmacist on the call and resolved the issue in just a few minutes.
Another director shared the story of a rep who had trouble reaching a doctor. Until then, this rep had only been able to reach the HCP's office staff but never the HCP themselves. Once, the office ran into an issue with managed care and needed the rep's help. While the rep was driving, she received a text from that office on Engage and pulled over to have a look. What she thought would be a text from the office staff was from the HCP. Since it was so easy to connect over the phone, the HCP simply installed the app to talk to the rep directly. Now, the HCP and rep speak to each other regularly.
Field leaders also expressed that there were no discernible trends behind the HCPs who were using inbound: HCPs from all age groups and regions were participating in chats. One leader's team even saw that one of their most frequent users was one of the oldest HCPs they worked with.
From the stories the field leaders told, both doctors and their office staff used inbound channels to connect with their reps. The setup worked well with how HCP offices operated: the office staff could message the rep to coordinate logistics and requests for the HCP, such as sample drops, office lunches, or in-person meetings. The HCP, on the other hand, could message the rep directly when they need information, such as when they want a piece of content, get stuck on insurance paperwork, or have a question about how a treatment works. The HCP's office staff would often tell each other about messaging their rep, which made it easy for the rep to build relationships with the HCP's entire practice.
"The more the field learned about the capabilities and connectivity that inbound offered, the more they wanted to go all in." Field sales director at a large biopharma
"We have a tagline for Engage: '24/7 concierge service.' It's how we advance the industry and create a great customer experience." Regional director at a large biopharma
The Next Evolution of the HCP Experience
With communication becoming increasingly digital and instantaneous in all aspects of our lives, it's hard to believe that the way HCPs speak with reps will remain the exception. And as some commercial leaders have said, HCPs are already pushing reps for instant messaging— the change is here, and it's inevitable. It's just a matter of whether the industry can embrace this change and take this opportunity to enhance how we collaborate with HCPs.
Compliant chat tools like Engage are more accessible now than ever and give your field team a suite of functionalities that expand how they can serve HCPs. With features for completing sample signatures, scheduling rep meetings, and sending educational content over compliant chat, Engage can give HCPs the same instantaneous service they would get with a consumer app while keeping your field teams in control of the conversation.
This model is the future of engagement: strong face-to-face relationships augmented by digital enablement. Through leveraging the existing rapport between HCPs and the field, reps can get more done for HCPs and build even deeper relationships tailored to each HCP's personalized needs. It elevates the transactional relationship between reps and HCPs into a partnership to get the right treatments into patients' hands faster.
Talk to your Veeva account team to learn about the latest innovations for customer-centric communication in Veeva Vault CRM Engage.
