White Paper
Mitigate Drug Shortage Risks by
Improving Internal and External Collaboration
Real-time access to unified data — shared across quality, regulatory, and production teams and with external contract partners — is key to reducing drug shortage risks.
Over the past decades, pharmaceutical manufacturing has become increasingly complex, and global supply chains more fragmented. Traditional approaches to data management have created silos that separate quality, regulatory, and manufacturing, preventing a more agile approach to manufacturing change control and batch release, and slowing data exchange and communication between sponsors and contract partners.
As a result, drug shortages have become more frequent1 and are now reaching record highs2 around the world. Currently, in the U.S., several oncology drugs are being rationed, while many workhorse antibiotics and analgesics are not available. According to a recent EU report3, three-quarters of EU member countries experienced inadequate supplies of key therapies. Meanwhile, the American Society for Hospital Pharmacists’ latest report found that 301 shortages occurred during the first quarter of 2023 alone, twice the number that had been seen in 20184.
For the past decade, as shortages have intensified, regulators and industry organizations have proposed risk-based measures to ensure adequate drug supplies. In 2018, during a national drug shortage crisis, the U.S. Food and Drug Administration (FDA) set up a task force that recommended incentives to spur investment in Quality Management Maturity (QMM), while the International Society for Pharmaceutical Engineering (ISPE) has studied best organizational practices for preventing shortages (Figure 1).
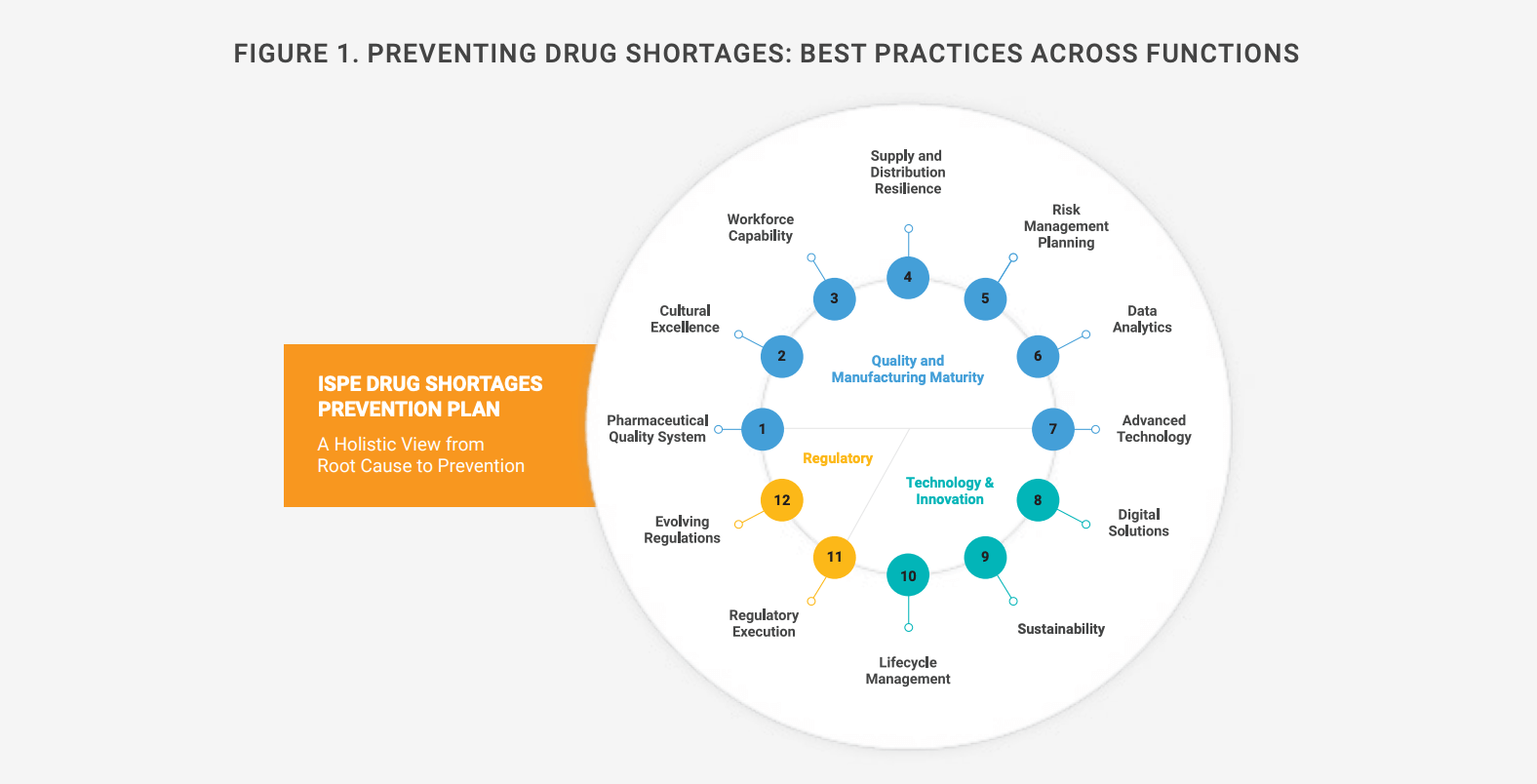
European regulators launched similar efforts in 2016 and issued guidance6 in 2023. Its goal is to help marketing authorization holders, wholesalers, distributors, and manufacturers optimize their response to drug shortages resulting from GMP noncompliance. It also includes best practices learned during the COVID-19 pandemic.
Evolving initiatives such as Identification of Medicinal Products (IDMP)7, Pharmaceutical Quality, Chemistry, Manufacturing & Controls (PQ/CMC)8, and ICH Q129 continue to address the need to standardize data and the exchange of information between stakeholders, to speed up communication and decision making to improve drug availability. They aim to enable FDA and the European Medicine Agency (EMA) to coordinate supply monitoring and management efforts. The European Community has also proposed regulatory reforms10 that would speed the commercialization of some generics and biosimilar products to bolster supplies.
Unifying data to reduce the risk of drug shortages
As drug supply gaps have worsened, the industry continues to increase its reliance on outsourcing, assigning strategic functions to outside partners to reduce risk, speed time to market, and gain access to specialized expertise and resources11. Successful partnerships demand clear communications and oversight to ensure process control, quality, and ultimately, trust.
The industry’s continued reliance on traditional legacy software has only intensified these challenges. Disconnected information silos require that quality, regulatory, and manufacturing teams use manual efforts and communication methods such as email to connect and manage processes and extend them to external partners. Relying on email to share documents and manage regulatory submissions, deviations, audit findings, and complaints with contract partners creates risks and hinders operational efficiency.
One way to avoid these problems is by investing in technology that enhances collaboration and the sharing of relevant data — both internally, across functions, and between sponsors and contract partners such as CDMOs and CROs. Cloud-based solutions gather information in one system and provide access to real-time quality, regulatory, and manufacturing data for all collaborators within and outside of a company’s boundaries (see Figure 2).
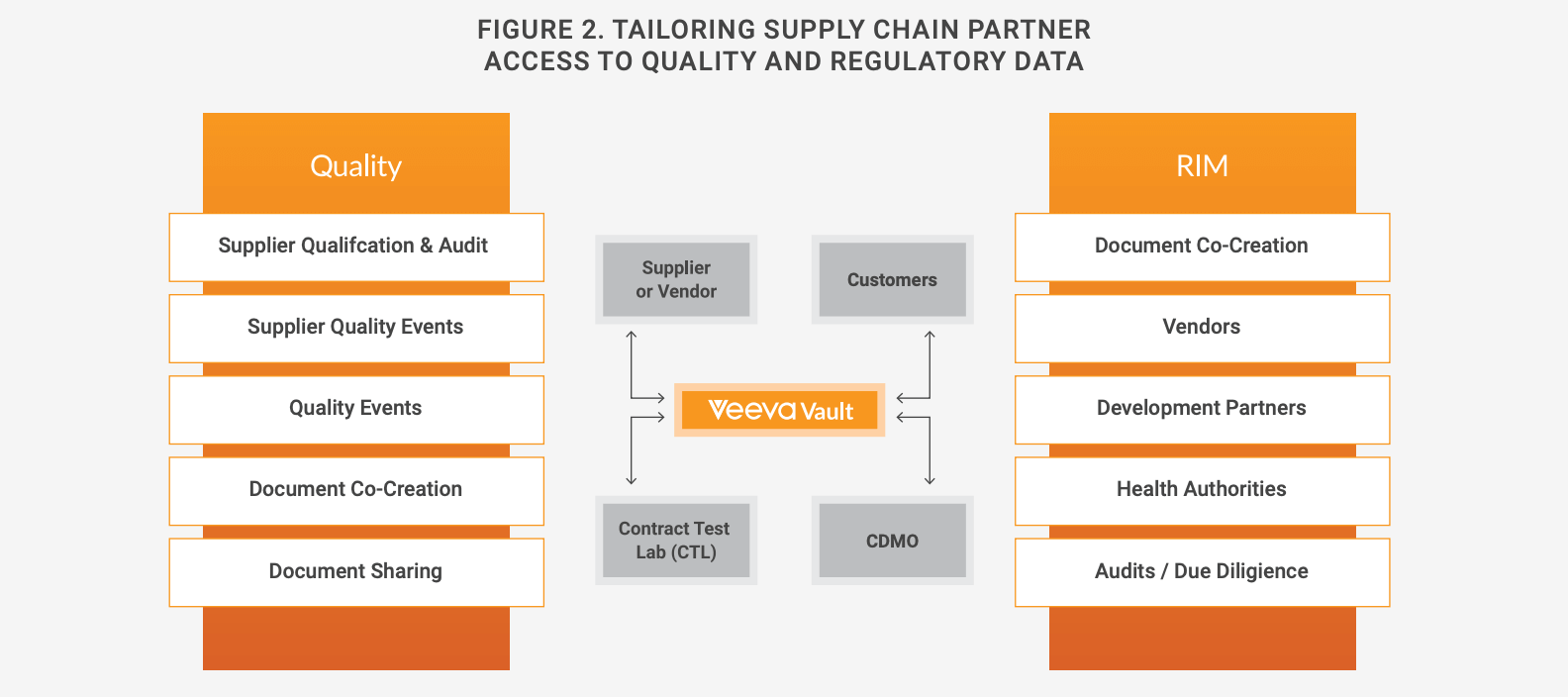
When configuring these applications, each company decides what data and documentation each of its external partners should have access to, in what form, and with what frequency. This ability to tailor how partners receive information guarantees security while giving contract partners access to the most relevant, up-to-date quality information directly from the sponsor’s quality management or document management system (QMS or DMS). It also allows companies to configure solutions so that external partners can add information directly into their digital systems, eliminating the need for emailing and rounds of verification, and reducing secondhand data-entry errors.
This approach is streamlining processes for sponsors and contract partners alike. Karyopharm Therapeutics is taking this approach with Vault QualityDocs to handle documentation with its suppliers. Using the application, vendors can upload relevant quality and regulatory documents directly into the sponsor’s DMS, eliminating manual data entry, verification, and rounds of emailing. “Vendor access is limited to what they can see, we don´t have to email documents, and we can find the documentation very easily,” says Maria Conklin, associate director of QC.
Meanwhile, a diversified pharma company has configured Vault QMS so that customers and suppliers alike can key information directly into the system for audit findings and corrective and preventive actions (CAPAs). Extending its QMS to partners has led to significant improvements in efficiency and decreased costs. The company is already working on extending this collaborative approach to complaints and deviations.
Expediting post-approval changes
Connected, cloud-based systems can also streamline the management of post-approval manufacturing changes, one of many root causes of drug shortages. Traditional approaches to change control use disconnected data, resulting in manual processes that delay batch release and product availability. They require frequent communication and the close coordination of efforts among production, quality, and regulatory teams.Each of these manual processes can entail hundreds of different steps and detailed interactions amongst cross-functional teams as they work to track down historical data and documents. For instance, if a manufacturing process or specification has changed, regulatory teams must see which countries and internal documents will be affected, while supply chain teams would need to look at data for individual product information. Using legacy approaches, it is difficult and time-consuming for teams to find and verify the information and documentation required.
Today, the typical large biopharma company contends with thousands of these changes globally each year — as many as 200 per product. Processing each change can take between six months and two years to complete, due to lack of visibility into separate data and limited integration between systems that include QMS, RIM, LIMS, and ERP.
The result is a significant amount of manual work, as well as redundant data and work steps, including meetings, phone calls, and emails. Connecting quality to RIM systems eliminates data disconnects and enables process automation, reducing the time and effort required for change management. Figure 3 shows how an integrated system works, including DMS and learning management systems.
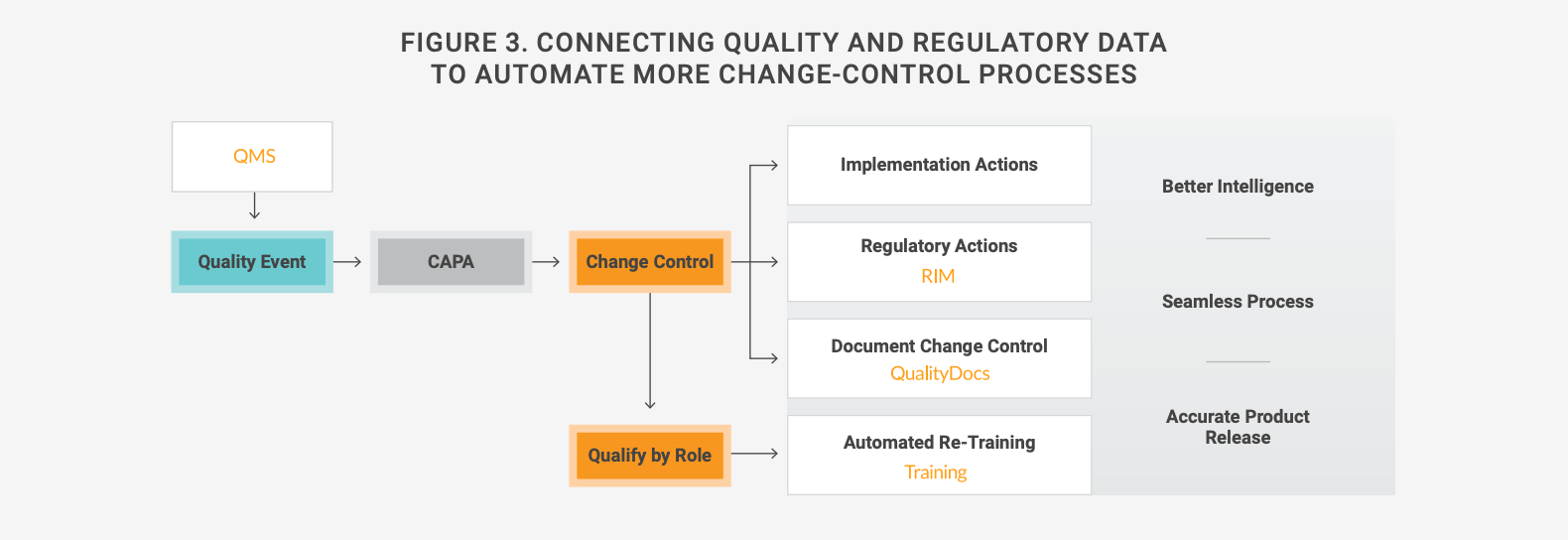
Among the companies adopting this approach is Moderna Therapeutics. Linking its regulatory and quality applications has led to significant reductions in the time needed for regional impact assessments and last-minute coordination with supply chain and regulatory departments. “Now, the data and information needn’t be requested or sit in someone’s inbox for two days,” notes Juhi Saxena, associate director of regulatory and clinical platforms.
Conclusion
There is no single remedy for drug shortages, but a growing number of companies are reducing risks and increasing agility by modernizing the way they approach quality and regulatory management, unifying across people, partners, and systems. Some are going one step further by connecting quality with regulatory data and documentation to eliminate manual processes. The result is better collaboration — both internally, across functions, and with external partners — to speed availability of treatments to the patients who need them.
To learn more about how your peers are using these approaches to improve operational excellence and minimize supply disruptions, and how Veeva is optimizing its quality and regulatory platform to meet these needs, please contact Sofia Lange, director of strategy for quality and manufacturing, or Vicki Cookson, enterprise strategy lead for regulatory affairs, at Veeva Systems.
See how Veeva streamlines change control.
1 U.S. Senate Committee on Homeland Security, “Short Supply: The Health and National Security Risks of Drug Shortages,” Final Report, June 6, 2023.
2 Lovelace, B. “One in Three US Hospitals Severely Affected by Drug Shortages, Survey Finds,” NBC News, August 10, 2023.
3 PGEU, “Medicine Shortages Survey Results,” 2022.
4 American Society of Hospital Pharmacists Webpage on Drug Shortages, 2022.
5 FDA, “Drug Shortages: Root Causes and Potential Solutions,” 2019. 6 EMA, “Regulatory Procedural Guidelines for the Prevention of Drug Shortages,” 2023.
7 EMA, “Data on Medicines (ISO IDMP Standards) Overview,” 2016.
8 FDA, “Pharmaceutical Quality – Chemistry, Manufacturing & Controls (PQ/CMC),” 2023.
9 EMA, ICH Q12 “Guidelines for Considerations of Pharmaceutical Product Lifecycle Management,” 2023.
10 EC, “Amendment to the Proposal for Regulation of Medical Products to be Authorized by the Union,” 2023.
11 “Predicted Growth in the Outsourcing R&D Sector”, TechTeamz.io, March 28, 2023.
