Features Brief
Submission Content Plans Feature Brief
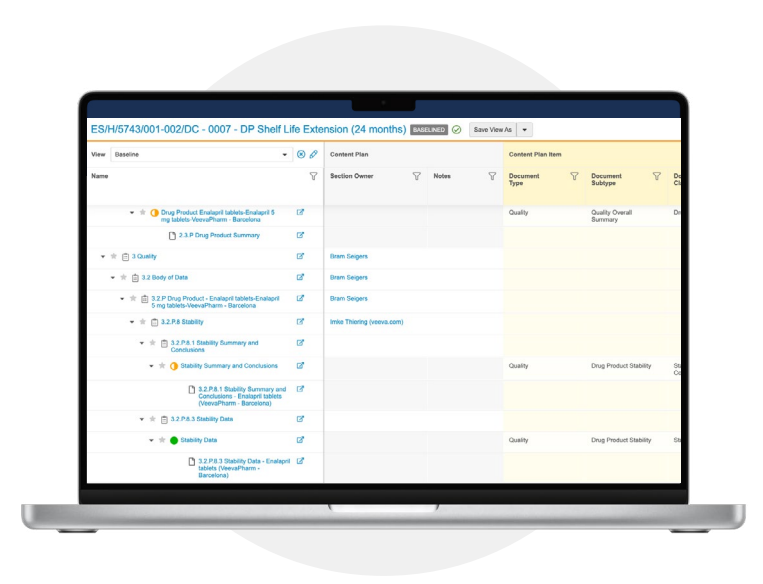
Simplify submission content planning, management, and compilation to save significant time and effort
Veeva RIM Submission Content Plans (SCPs) provide a unique, structured, and collaborative approach to planning, tracking, and managing submission development. As the volume of submissions continues to grow, regulatory teams need a way to track submission development across multiple markets. Many companies are using multiple tools for this work, including Excel, Microsoft Project, emails, and file shares. With SCPs, teams can efficiently plan, track, and develop all content required for submissions in a single, collaborative space.

Business Benefits
-
Increase submission efficiency.
Streamline communication and task delegation with a single source of truth, while reducing duplicative tasks and manual data entry with templates and automation.
-
Transparent global submission statuses.
Gain a holistic, real-time, view of individual document and submission progress across submission teams and markets.
-
Enhanced compliance.
Improve global traceability of submitted documents, including what versions were submitted to which Health Authorities, and where to find the latest source documents when it’s time to make an update.
Features
-
Content plan templates
Leverage our recommended constraints to standardize submission planning by submission type, product, or region.
-
Content plan viewer
Plan submissions, collaborate on content assembly, and track progress to completion using a holistic view that can be easily tailored to each user’s preference.
-
Advanced document matching
Reduce manual tasks with Veeva’s most recent improvements to automated and suggested matching for documents.
-
Drag-and-drop documents
Drag and drop RIM documents from your library, or external files from your desktop for automated assignment and document upload.
-
Real-time reports and dashboards
Automate status reporting on progress, tasks, and timelines with real-time dashboards and reports. Easily report on which documents have been submitted to which Health Authorities.
-
Foundation for Continuous Innovation
SCPs is a prerequisite for adopting more of Veeva RIM’s high value features, including Active Dossier, Global Content Planning and Veeva Publishing.
Veeva RIM
Submission Content Plans (SCPs) are a capability within Veeva RIM that streamlines submission preparation on a single, cloud-based platform. SCPs enable companies to:
- Develop reliable regulatory content with high data integrity
- Coordinate regulatory efforts across internal teams, affiliates, partners, and regions
- Increase end-to-end process efficiency from submission planning to publishing
Learn more about how Veeva Submissions can streamline your global regulatory submission process.
