Veeva Pulse Reveals Digital Content More Than Twice as Effective in Driving Promotional Response
Significant opportunity for biopharmas as only 29% of HCP meetings today present digital content
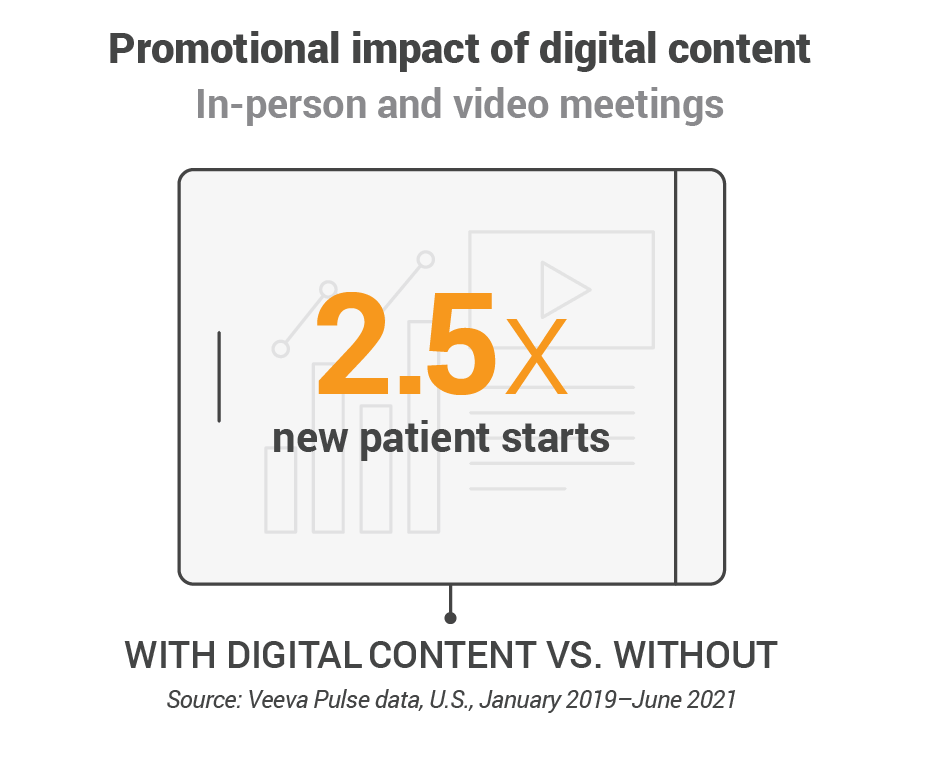
BARCELONA, Spain — 6 Apr. 2023 — Veeva Systems (NYSE: VEEV) today released the latest findings from its Veeva Pulse Field Trends Report, the largest analysis of global healthcare professional (HCP) engagement across the biopharma industry. Data show that leveraging digital content during in-person and video meetings more than doubles the promotional response over those that do not use digital content.
Using digital content in HCP meetings has a significant, measurable impact. Veeva Pulse insights show successful field teams share content four times more than companies that don’t, creating a clear advantage. Yet, more than 60% of field engagements across the industry don’t use digital content, missing an opportunity to make the most of limited and timely exchanges with HCPs.
Veeva Pulse analysis shows digital content used during meetings is a key lever to increase field effectiveness and drive more impactful conversations:
- Digital content boosts promotional response. U.S. data show a global trend that digital content used during video and in-person meetings more than doubles the promotional response over meetings that don’t share content.
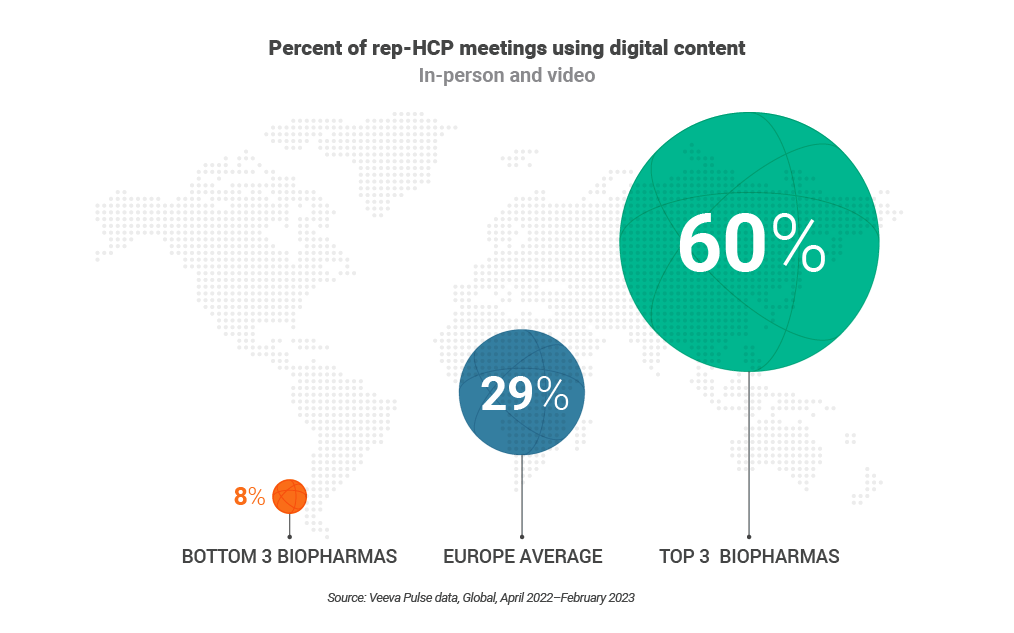
- Successful teams use content four times more. European field reps that use digital content the most outpace four times others who don’t frequently leverage it. Field teams have room for growth, currently sharing digital content in just 29% of meetings, despite its proven effectiveness.
- High-impact content drives HCP engagement. In the last year, biopharmas created more content. Yet, of all the content created, 76% is rarely or never used. This indicates a need for companies to focus their content strategy on developing fewer assets that are proven effective in advancing relevant engagements with HCPs.
“We continue to see the impact digital content has in driving better sales outcomes and building more personalized, connected HCP experiences, whether in-person or virtual,” said Aaron Bean, vice president of Veeva business consulting, Europe. “Companies who use their engagements as a chance to inform HCPs with high-impact content will gain an advantage and create new opportunities for follow-up conversations.”
About the Veeva Pulse Field Trends Report
Analyzing over 600 million HCP interactions and activities annually from more than 80% of commercial biopharma field teams worldwide, the Veeva Pulse Field Trends Report is the largest industry benchmark of its kind on HCP engagement. The analysis compiles real-time transactional data recorded in Veeva CRM globally and from European markets including the United Kingdom, Italy, Germany, France, and Spain. Indexed by Veeva quarterly, the data will help companies effectively and accurately benchmark performance to set the right, actionable goals for continued growth and impact.
Additional Information
To download a copy of the Veeva Pulse Field Trends Report, visit: veeva.com/eu/FieldTrends
Learn more about Veeva Business Consulting: veeva.com/eu/BusinessConsulting
Connect with Veeva on LinkedIn: linkedin.com/company/veeva-systems
About Veeva Systems
Veeva is the global leader in cloud software for the life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 1,000 customers, ranging from the world’s largest pharmaceutical companies to emerging biotechs. As a Public Benefit Corporation, Veeva is committed to balancing the interests of all stakeholders, including customers, employees, shareholders, and the industries it serves. For more information, visit veeva.com/eu.
Veeva Forward-looking Statements
This release contains forward-looking statements regarding Veeva’s products and services and the expected results or benefits from use of our products and services. These statements are based on our current expectations. Actual results could differ materially from those provided in this release and we have no obligation to update such statements. There are numerous risks that have the potential to negatively impact our results, including the risks and uncertainties disclosed in our filing on Form 10-K for the fiscal year ended January 31, 2023, which you can find here (a summary of risks which may impact our business can be found on pages 9 and 10), and in our subsequent SEC filings, which you can access at sec.gov.
Contact
Jeremy Whittaker
Veeva Systems
+49-695-095-5486
jeremy.whittaker@veeva.com
