Veeva SafetyDocs
Gain Control Over Your Safety Processes
Efficiently collaborate and improve compliance globally
Centrally Manage Pharmacovigilance
Processes and Content
Easily manage safety-related processes and content in one validated solution designed for pharmacovigilance.
-
Increase Operational Efficiency
Automate processes, streamline document management, and centralize content
-
Enable Global Collaboration
Securely share content and assign tasks with controlled workflows internally and externally
-
Improve Inspection and Audit Readiness
Gain real-time visibility and oversight, and always use approved content
Solutions
Veeva SafetyDocs
Harmonize content and processes with controlled workflows and flexible security to drive greater operational efficiency and compliance.
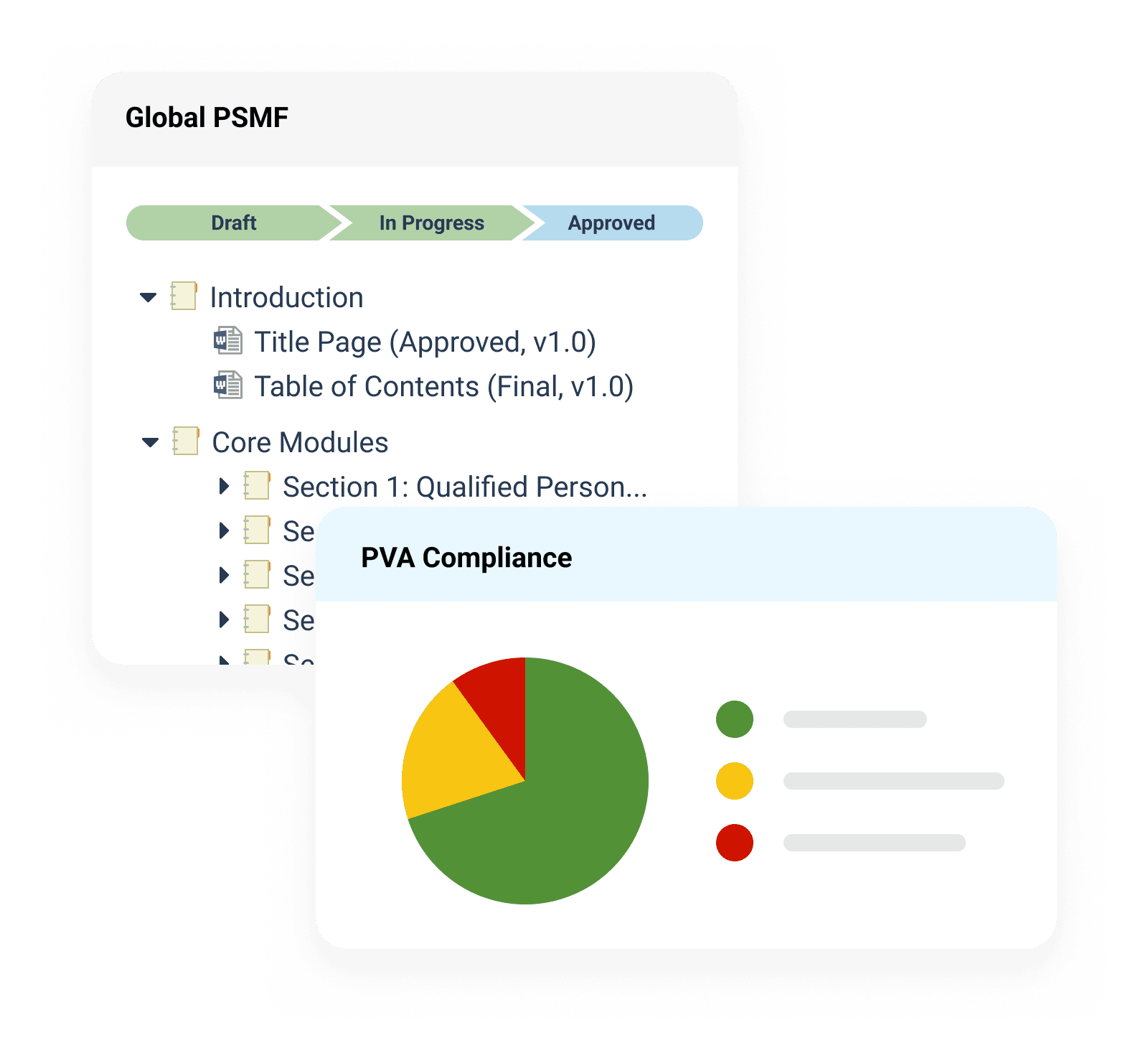
PSMF Management
Centrally manage global Pharmacovigilance System Master Files (PSMF) and easily stay inspection-ready.
Learn More
PVA Management
Seamlessly oversee the status of pharmacovigilance agreements (PVAs) and partner obligations.
Watch Demo
Risk Management
Author, manage, and track core and local risk management plans (RMP) and additional risk minimization measures (aRMMs).
Watch Demo
Literature Management
Streamline literature review processes and identify possible ICSRs and safety signals faster.
Watch Demo
Aggregate Reports
Simplify the assembly and authoring of aggregate reports for better oversight and compliance.
Watch Demo
Signal Management
Investigate potential safety signals, manage relevant documentation, and maintain compliance.
Watch Demo