Article
Optimize MLR Workflows Using a Central Claims Library
Content is vital in life sciences marketing. Done well, it builds trust in your brand, informs targets about cutting-edge science and technology, and showcases your organization to the right audiences. As a result, requests for content keep rising, meaning marketers have more to create, approve, maintain, and catalog — not to mention deliver metrics on what assets are being used and which are making the biggest impact.
Scaling content requires you to address how assets typically live in a document, reference, claims, or graphics library. A digital asset management (DAM) solution connects the libraries so you can monitor the medical, legal, and regulatory (MLR) approvals for every element in them. In addition, maintaining a central, digital repository helps to meet increased content demand and new or changing compliance requirements.
With that foundation, let’s focus on claims libraries and the MLR processes associated with them.
The case for a centralized claims library
A central claims library enables you to quickly yet compliantly publish materials that field reps and others request. It’s a difficult and high-stakes act to greenlight the use of assets when it’s unclear which claims are approved. If claims live in multiple places, the problem of time-consuming or confusing MLR workflows is exacerbated, making it harder for teams to get the content they need on their timelines. And worse, organizations that lack an organized claims library risk FDA false claims notifications that can carry punitive damages.
On the other end of the spectrum, life sciences organizations with centralized claims management and optimal MLR workflows have seen a 57% reduction in review cycle times and a 55% drop in time spent in review meetings in Veeva’s experience. Plus, they have empowered teams who can count on quickly providing compliant content to HCPs and others when it’s needed because:
- Internal and/or contracted MLR review teams know where to go for approved text and references, saving time and resources.
- Teams don’t need to link and review references over and over.
- Claims and reference anchors get approved once as a claim record accessible to all.
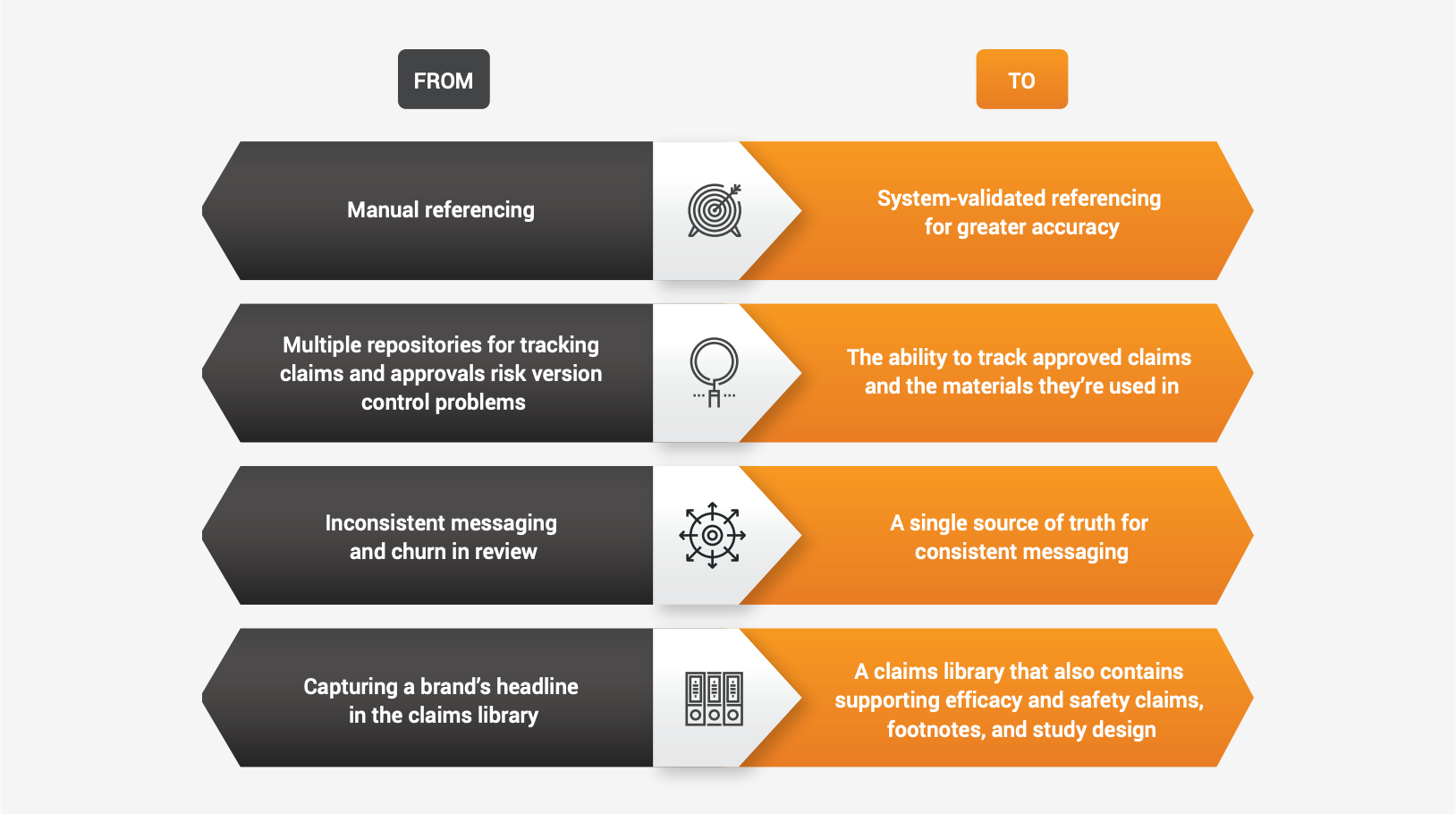
Automation’s role in optimizing claims
Implementing modern claims management processes means automating at least some portions of the claims management process — for example, auto-linking claims to references in a claims library, as Lundbeck did. In general, introducing technology to manage claims brings benefits such as:- Reduced reliance on paper and spreadsheets
- Streamlined and standardized workflows that save time
- A seamless process for providing claims information
- Increased efficiency in onboarding new personnel
Beyond gaining efficiency, automation can help life sciences organizations better mitigate false claims risks, increase claims use, ensure access to current claims data, and optimize resource allocation.
Ready to optimize MLR workflows?
Your goals are to gather and approve high-quality content submissions in one place, in less time, to publish better content. Once you’ve established a central — ideally digital — claims library or a DAM for all assets, you can focus on improving MLR workflows. But remember: by nature, the MLR process is slow and steady, so your workflow optimization of it will be, too. Start small and build on incremental wins to demonstrate progress and promote buy-in.
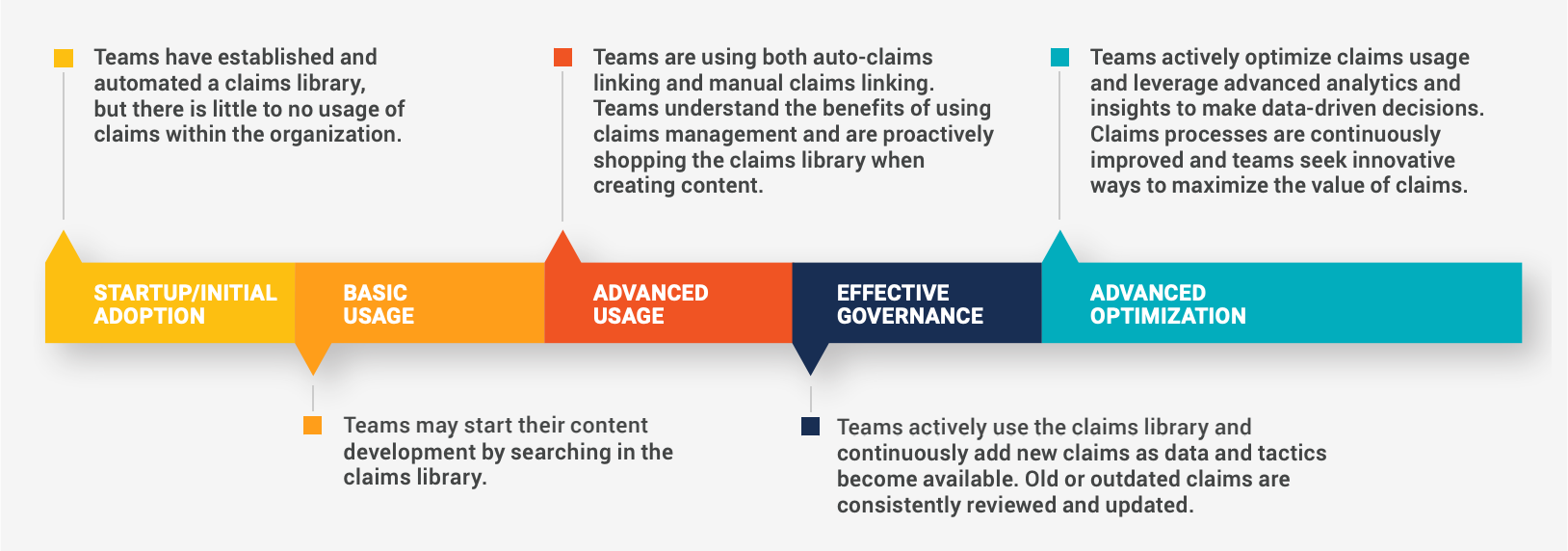
In addition, your organization’s path to creating or optimizing your claims library and MLR processes will be unique. However, the following general framework helps gauge your starting point and aids in auditing progress through general milestones, from initial adoption to advanced optimization.
Evangelize the project
Creating or revamping your central claims library and MLR processes requires crucial conversations with the right stakeholders. Ideally, you’ll foster their buy-in and they, in turn, spur interest and alignment among others affected by the changes. For these essential, “what’s in it for you” conversations, here’s a quick summary of how various stakeholders and teams benefit from a claims library:
1. COMMERCIAL LEADERS
- Reduce organizational spending
- Increase speed to market
- Reduce challenges with MLR processes
2. REVIEWERSS
- Increase regulatory confidence in claim links from the library
- Reduce time reviewing for a greater focus on content
- Create a single source of truth for documented approvals
3. MARKETERS/AGENCY OF RECORDS
- Increase review efficiency
- Create a single source of truth for documented approvals
- Decrease agency spend
- Increase review speed through MLR
- Decrease timelines for derivative content/increase use of production partners for derivative materials
- Increase resources for more creative/strategic thinking
4. MARKETING OPERATIONSS
- Reduce time in review meetings
- Decrease back and forth with agencies and MLR teams
- Increase efficiency between team operations
- Decrease suggestions from editors for reduced churn
- Reduce the number of annotations to review
The need for a central, regulated claims library that enables the creation, review, and approval of claims and content is growing rapidly along with the volume of requests for life sciences content. By making content and claims easily accessible for users, you’re enhancing their ability to reuse approved, compliant content using optimized MLR workflows that make your job more efficient, too.
Discover more content best practices at life sciences companies.
